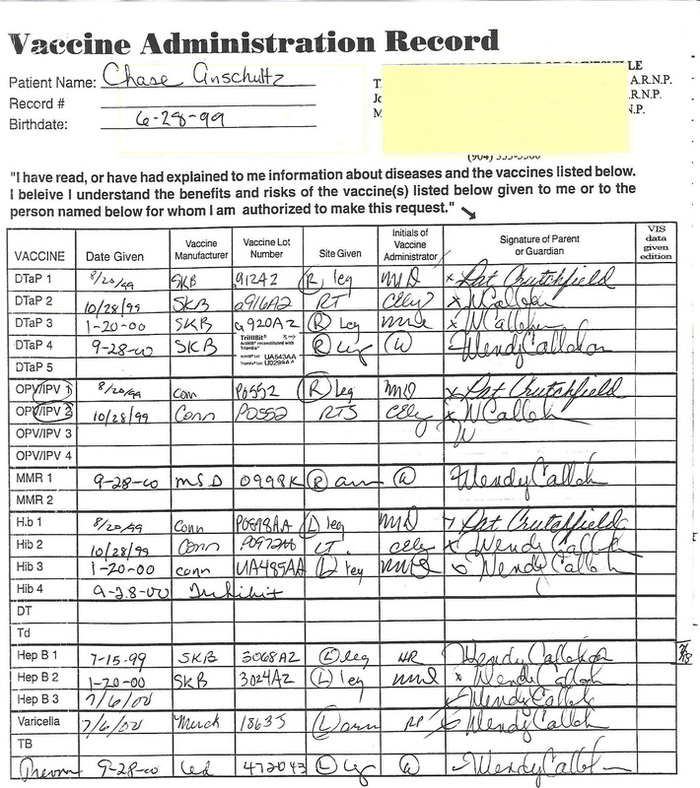
Lot number
DTaP ......UO29944
No adverse events found
MMR.......0998K
Adverse events:
DTaP ......UO29944
No adverse events found
MMR.......0998K
Adverse events:
Vaccinations Manufacturer Lot Dose Route Site
1 MMR MSD 0998K 1 SC LL
2 PNC LEDERLE 472042 0 IM RL
1 MMR MSD 0998K 1 SC LL
2 PNC LEDERLE 472042 0 IM RL
Onset Date: 2000-09-30 Number of Days: 1 Symptoms: HYSN INJECT SITE MASS INJECT SITE PRURITUS Redness, pruritus and induration 8cm X 7cm. Treated with Prelone and Augmentin.
Vaccinations Manufacturer Lot Dose Route Site
HEP SMITHKLINE ENG3232A4 1 IM LA
2 IPV CONNAUGHT LTD R1472 0 IM RA
3 MMR MSD 0998K/0660 1 SC LA
4 TD CONNAUGHT LABS U0265AA 1 IM RA
Onset Date: 2000-10-11
Number of Days: 0 Symptoms: RASH MAC PAP TWITCH
15 minutes, post vax, pt noticed right arm twitching. On exam; a local rash was noted on his right and left upper arm (papular rash). No further problems occurred in the following 1 week.
Vaccinations Manufacturer Lot Dose Route Site
HEP SMITHKLINE ENG3232A4 1 IM LA
2 IPV CONNAUGHT LTD R1472 0 IM RA
3 MMR MSD 0998K/0660 1 SC LA
4 TD CONNAUGHT LABS U0265AA 1 IM RA
Onset Date: 2000-10-11
Number of Days: 0 Symptoms: RASH MAC PAP TWITCH
15 minutes, post vax, pt noticed right arm twitching. On exam; a local rash was noted on his right and left upper arm (papular rash). No further problems occurred in the following 1 week.
The Age of Autism: Adverse events
By DAN OLMSTED
Years before the alarm sounded nationwide about a possible link between vaccines and autism, some doctors were making that connection themselves.
The evidence: 83 reports filed with the Vaccine Adverse Event Reporting System associating the onset of autism with childhood immunizations. The reports, compiled and catalogued by the Centers for Disease Control and Prevention and the Food and Drug Administration, were analyzed by Age of Autism.
A report from 1992 listed Feb. 21 as both "vaccination date" and "adverse event date" for a 1-year-old boy: "Patient received MMR vaccination (measles-mumps-rubella) and experienced fever, autistic behaviors, encephalitic condition, began to tune out; sound sensitivity, hand-flapping, wheel-spinning, nighttime sweats, appetite increase."
The child's diagnoses included autism, encephalopathy (brain swelling), mental retardation, personality disorder and speech disorder.
Another report: Two days after being vaccinated in August 1994 a 1-year-old girl experienced "low fever, much discomfort. Patient laid in bed and cried and moaned; three-four days post-vaccination, rash traveled over patient's body and lasted at least one week. Within six weeks of vaccination patient was observed as losing previously gained language and social skills; diagnosed autistic."
The reports do not prove that any of the autism cases resulted from vaccination. Rather, their potential significance is that a number of qualified observers -- primarily doctors and other health professionals -- suspected a connection and made the effort to report it well before the issue was on the national radar.
In July 1999 the CDC and the American Academy of Pediatrics recommended that manufacturers begin phasing out a mercury-based preservative that was in several childhood vaccines. The concern: As the number of required vaccinations expanded around 1990, children inadvertently got too much mercury, a known neurotoxin.
Since then federal health officials, along with a panel of the Institute of Medicine, have dismissed the concern as unfounded. But some scientists and parent groups continue to assert that childhood immunizations are behind a major rise in autism diagnoses.
The adverse-event reports examined by Age of Autism were sent to VAERS between June 1991 and June 1999 -- the month before the CDC recommendation to phase out thimerosal.
Based on a 1994 report by a California physician, 10 of the 83 cases are unknown children "who received vaccination and (were) diagnosed with autism and encephalopathy." That doctor reported "there are currently 10 cases of autism in children who received DPT/OPV/MMR at 15-18 months." (The reference is to the diphtheria-pertussis-tetanus, polio and MMR vaccines.)
That report also cites a statement from an unidentified vaccine manufacturer: "Dr. ... is not treating physician and does not possess any original records; unclear whether reporter is suggesting possible causal association."
The following excerpts start with the date the report was received by VAERS and the age of the child when vaccinated. The type of vaccine is not always clear and is indicated here only when specified in the event narrative. Medical abbreviations are spelled out for clarity.
-- June 5, 1991, age 1.5. Four days post-vaccination developed running fever. To emergency room, temperature 104 and very lethargic. Several tests for various things and couldn't find anything wrong; given antibiotics as a precaution; then lost speech and was diagnosed with autism.
-- July 7, 1995, age 1.9. Patient developed localized swelling with redness in injection site following vaccination. Also had high temperature, loss of balance, limping followed with high-pitched screaming and loss of speech. Diagnosed pervasive personality disorder/autistic.
-- May 29, 1996, age 1.3. Patient received vaccination, experienced a fever of 104.6 which lasted for approximately three to four days. Patient was very lethargic and appeared changed in temperament and abilities; symptoms of autism such as head banging were noticed.
-- April 3, 1997, age 1.2. Evening of vaccination, patient developed temperature of 104.2 and cried all night with high pitched screaming. (Next day) patient wouldn't eat and was listless; patient went off all solid foods; within next three months patient lost all speech abilities; diagnosed with autism.
-- April 15, 1997, age 1.3. Patient received vaccination and developed a big change in mental status that was described as an atypical infantile autistic state with mild seizures; at the age of one or two the patient started sleeping all the time.
-- Aug. 4, 1997, age 6 months. Patient received vaccination and immediately experienced a fever of 104 and developed a quarter-size lump at the injection site; 30 to 60 days post-vaccination patient developed autism; the quarter-size lump persisted for approximately six weeks.
-- March 2, 1999, age 1.3. Loss of all developmental milestones immediately post-vaccination; patient has autism; communication disorder, auditory processing disorder, asthma, food allergies, chronic diarrhea, digestive problems.
-- April 21, 1999, age 1.5. Patient received vaccination; (three days later) after midnight patient woke up screaming unconsolably with hysteria. Later in morning, patient could not talk and could not comprehend anything. Extensive testing done; diagnosis: autism. Continues in this state today.
-- May 27, 1999, age 1.3. Fever immediately post-vaccination -- diarrhea, ulcers on diaper area; chronic digestive problems, loss of speech; stimming behavior; autism, seizure disorder. The patient's ulcerative colitis and gastritis are currently under the care of a physician.
Since 1999, the federal government's vaccine databases have come under scrutiny from critics who charge they have been manipulated to show no connection between vaccines and autism. They also say the CDC has a built-in conflict of interest because it sets the universal childhood immunization schedule that is then adopted by the states.
The agency vigorously defends its research and denies that its role in vaccination policy compromises its objectivity.
Last week Sen. Joseph Lieberman, D-Conn., said he plans to seek funding for an independent review of another CDC vaccine database.
"Part of what I'm going to require in this amendment we're going to put up is that the independent studies not only look at the data, but actually talk to some of the -- examine some of the kids and families to go over family histories," Lieberman said.
This ongoing series on the roots and rise of autism welcomes reader comment. E-mail: dolmsted@upi.com
Copyright 2005 by United Press International. All Rights Reserved.
By DAN OLMSTED
Years before the alarm sounded nationwide about a possible link between vaccines and autism, some doctors were making that connection themselves.
The evidence: 83 reports filed with the Vaccine Adverse Event Reporting System associating the onset of autism with childhood immunizations. The reports, compiled and catalogued by the Centers for Disease Control and Prevention and the Food and Drug Administration, were analyzed by Age of Autism.
A report from 1992 listed Feb. 21 as both "vaccination date" and "adverse event date" for a 1-year-old boy: "Patient received MMR vaccination (measles-mumps-rubella) and experienced fever, autistic behaviors, encephalitic condition, began to tune out; sound sensitivity, hand-flapping, wheel-spinning, nighttime sweats, appetite increase."
The child's diagnoses included autism, encephalopathy (brain swelling), mental retardation, personality disorder and speech disorder.
Another report: Two days after being vaccinated in August 1994 a 1-year-old girl experienced "low fever, much discomfort. Patient laid in bed and cried and moaned; three-four days post-vaccination, rash traveled over patient's body and lasted at least one week. Within six weeks of vaccination patient was observed as losing previously gained language and social skills; diagnosed autistic."
The reports do not prove that any of the autism cases resulted from vaccination. Rather, their potential significance is that a number of qualified observers -- primarily doctors and other health professionals -- suspected a connection and made the effort to report it well before the issue was on the national radar.
In July 1999 the CDC and the American Academy of Pediatrics recommended that manufacturers begin phasing out a mercury-based preservative that was in several childhood vaccines. The concern: As the number of required vaccinations expanded around 1990, children inadvertently got too much mercury, a known neurotoxin.
Since then federal health officials, along with a panel of the Institute of Medicine, have dismissed the concern as unfounded. But some scientists and parent groups continue to assert that childhood immunizations are behind a major rise in autism diagnoses.
The adverse-event reports examined by Age of Autism were sent to VAERS between June 1991 and June 1999 -- the month before the CDC recommendation to phase out thimerosal.
Based on a 1994 report by a California physician, 10 of the 83 cases are unknown children "who received vaccination and (were) diagnosed with autism and encephalopathy." That doctor reported "there are currently 10 cases of autism in children who received DPT/OPV/MMR at 15-18 months." (The reference is to the diphtheria-pertussis-tetanus, polio and MMR vaccines.)
That report also cites a statement from an unidentified vaccine manufacturer: "Dr. ... is not treating physician and does not possess any original records; unclear whether reporter is suggesting possible causal association."
The following excerpts start with the date the report was received by VAERS and the age of the child when vaccinated. The type of vaccine is not always clear and is indicated here only when specified in the event narrative. Medical abbreviations are spelled out for clarity.
-- June 5, 1991, age 1.5. Four days post-vaccination developed running fever. To emergency room, temperature 104 and very lethargic. Several tests for various things and couldn't find anything wrong; given antibiotics as a precaution; then lost speech and was diagnosed with autism.
-- July 7, 1995, age 1.9. Patient developed localized swelling with redness in injection site following vaccination. Also had high temperature, loss of balance, limping followed with high-pitched screaming and loss of speech. Diagnosed pervasive personality disorder/autistic.
-- May 29, 1996, age 1.3. Patient received vaccination, experienced a fever of 104.6 which lasted for approximately three to four days. Patient was very lethargic and appeared changed in temperament and abilities; symptoms of autism such as head banging were noticed.
-- April 3, 1997, age 1.2. Evening of vaccination, patient developed temperature of 104.2 and cried all night with high pitched screaming. (Next day) patient wouldn't eat and was listless; patient went off all solid foods; within next three months patient lost all speech abilities; diagnosed with autism.
-- April 15, 1997, age 1.3. Patient received vaccination and developed a big change in mental status that was described as an atypical infantile autistic state with mild seizures; at the age of one or two the patient started sleeping all the time.
-- Aug. 4, 1997, age 6 months. Patient received vaccination and immediately experienced a fever of 104 and developed a quarter-size lump at the injection site; 30 to 60 days post-vaccination patient developed autism; the quarter-size lump persisted for approximately six weeks.
-- March 2, 1999, age 1.3. Loss of all developmental milestones immediately post-vaccination; patient has autism; communication disorder, auditory processing disorder, asthma, food allergies, chronic diarrhea, digestive problems.
-- April 21, 1999, age 1.5. Patient received vaccination; (three days later) after midnight patient woke up screaming unconsolably with hysteria. Later in morning, patient could not talk and could not comprehend anything. Extensive testing done; diagnosis: autism. Continues in this state today.
-- May 27, 1999, age 1.3. Fever immediately post-vaccination -- diarrhea, ulcers on diaper area; chronic digestive problems, loss of speech; stimming behavior; autism, seizure disorder. The patient's ulcerative colitis and gastritis are currently under the care of a physician.
Since 1999, the federal government's vaccine databases have come under scrutiny from critics who charge they have been manipulated to show no connection between vaccines and autism. They also say the CDC has a built-in conflict of interest because it sets the universal childhood immunization schedule that is then adopted by the states.
The agency vigorously defends its research and denies that its role in vaccination policy compromises its objectivity.
Last week Sen. Joseph Lieberman, D-Conn., said he plans to seek funding for an independent review of another CDC vaccine database.
"Part of what I'm going to require in this amendment we're going to put up is that the independent studies not only look at the data, but actually talk to some of the -- examine some of the kids and families to go over family histories," Lieberman said.
This ongoing series on the roots and rise of autism welcomes reader comment. E-mail: dolmsted@upi.com
Copyright 2005 by United Press International. All Rights Reserved.