ASU researcher receives grant to develop cancer vaccine
The Business Journal of Phoenix - July 10, 2007
by Angela Gonzales
http://www.bizjournals.com/phoenix/stories/2007/07/09/daily15.html?from_rss=1
A researcher at Arizona State University's Biodesign Institute in Tempe received $7.5 million from the U.S. Department of Defense to develop a cancer vaccine.
Stephen Albert Johnston, director of the institute's Center for Innovations in Medicine, will focus his research project on breast cancer. He is one of only two recipients in the nation to receive a five-year, $7.5 million grant from the DoD's Innovator Award, funded through its Breast Cancer Research Program.
The money will be used to pay for preclinical tests using mice and human tumor tissue samples from Mayo Clinic to make sure his idea will work, Johnston said. Once he gets past that point, he would collaborate with Mayo Clinic in Scottsdale to test the vaccine on people in phase one clinical trials. These most likely would be healthy people who are predisposed for having cancer, and would test for any reactions, such as autoimmune responses like lupus.
Johnston said the most frustrating part about his work is that it normal cells are more complicated than he expected. "It's harder to find the difference between tumor and normal cells than we thought it would be," he said. "Nobody knew normal cells were as complicated as they are. It's a good basic discovery, but from a practical point of view, it seems we're going to have to work harder."
Cancer is the second leading cause of death in the United States, with an estimated 1.45 million cases of cancer diagnosed this year. More than 559,000 people will die from the disease this year. Breast cancer is the second leading cause of death in women.
The DoD is using appropriations from a congressionally directed medical research program to eradicate breast cancer.
Johnston's goal is to make a vaccine that could be given to all adult women to prevent the occurrence of breast cancer in the same way vaccines have been created against infectious diseases.
Cancer isn't only a national problem, he said, but is rampant in Third World countries, where people don't have the technologies and finances to fight the devastating disease, he said. "Most of the people who die of cancer are not in the industrialized world," Johnston said, estimating that 70 percent of the cancers are in the developing world. He said the average expense for the first year of cancer treatment is about $17,000.
Johnston, who was recruited from University of Texas Southwestern Medical Center in Dallas, cautions he has a long way to go.
"This is a high risk deal," he said. "We have yet to see whether the basic idea will work. We're just glad some people thought it was worth exploring."
http://www.timesonline.co.uk/tol/life_and_style/health/article1873020.ece
From The Times
June 2, 2007
Flu-jab alert prompts study to see if vaccines could harm unborn babies
David Rose
Scientists are to investigate how vaccinations given to pregnant women might affect the health of their unborn child, after research suggested that babies’ immune systems develop much earlier than thought. A study published in the US Journal of Clinical Investigation yesterday found that the children of mothers who were given vaccinations against influenza started producing immune cells to combat the illness while still in the womb.
It is unclear whether such early production of antibodies has adverse or positive effects on an infant’s health.
Some researchers have suggested that exposure to vaccines, pollens and other agents during pregnancy may increase a child’s chances of developing allergies later in life. Such a hypothesis has been cited as the reason for rising rates of asthma and related illnesses. Vaccinating pregnant women against flu is currently considered safe and the Department of Health is considering whether to implement recommendations made in December, by the Joint Committee on Vaccines and Immunisation (JCVI), the official watchdog, that all pregnant women be given the jabs when elderly and vulnerable patients are vaccinated during the winter flu season.
A team of researchers from Columbia University, New York, studied 126 women who were given flu vaccinations, which are already recommended for all mothers-to-be in the United States.
Specific antibodies found in the umbilical cord of their babies suggest that proteins contained in the jabs passed from mother to foetus, and stimulated production of immune cells in the developing child. Antibodies were found in approximately 40 per cent of the cord blood samples, suggesting that the infants’ immune systems were capable of responding to agents passed from mother to child.
Previously, babies were thought to derive antibodies to protect them against illness from their mothers, via the placenta, not developing their own immune responses until some weeks after birth. “These results have important implications for determining when immune responses to environmental exposures begin,” Rachel Miller, the lead author of the paper, said. “More research now needs to be done on what the effect to the child in later life is.”
Professor Miller said: “It is possible that the early stimulation of a child’s immune system might lead to the child developing asthma, eczema or other illnesses, but it is also possible that the beneficial effect of the vaccine might be conferred from mother to child, and protect the baby in early life.”
Donald Peebles, a consultant from University College London, and spokesman for the Royal College of Obstetricians and Gynaecologists, said that it was known that viruses and other agents could pass from mother to a developing baby, but more research was needed to determine the potential health effects.
“This study shows that the foetus is a good deal more sophisticated in developing its own immune responses than previously thought,” he said.
Immune System Research Hold Promise For Alzheimer's, Stroke, And mental Disorders
ScienceDaily (Nov. 7, 2007) —
http://www.sciencedaily.com/releases/2007/11/071106124045.htm
Recent discoveries in the field of neuroimmunology, which studies the interaction between the immune and nervous systems, are offering promising new leads for the treatment of many devastating neurological disorders, from Alzheimer's disease to stroke. New research suggests that reducing the expression of an immune system protein in the brain may help repair neurons damaged by spinal cord injury and other trauma. Other research has uncovered the important role that immune molecules perform in the prenatal development of such diseases as autism and schizophrenia. Additional findings reveal that an innovative type of immunotherapy assists with the recovery of memory after stroke.
"The discovery that immune molecules play a crucial role in shaping neuronal connections and are even expressed on nerve cells important in learning and memory is opening up a whole range of potential new treatment targets for diseases in which these connections have gone awry, such as Alzheimer's and other dementias, autism, amyotrophic lateral sclerosis (ALS), Parkinson's disease, schizophrenia, and in nerve injury," says Esther Sternberg, MD, of the National Institutes of Health. "Understanding these neural immune connections at a molecular and cellular level will shed light on the reasons these diseases develop and will help provide new ways to prevent or treat them."
Several years ago, researchers at Harvard Medical School made the unexpected discovery that neurons have major histocompatibility complex (MHC) class I molecules on their cell surface. MHC class I molecules play a central role in a healthy, functioning immune system by helping the body recognize and destroy disease-infected cells.
"We were amazed by this finding," says Carla Shatz, PhD, now at Stanford University. "Previously it had been thought that neurons were the only cells in the body that didn't express these molecules." When Shatz and her colleagues studied mouse models that lack MHC class I, they found another surprise: greater-than-normal strengthening of the synapses between neurons. This observation suggests that MHC class I acts as a kind of "molecular brake" on synaptic plasticity, the ability of brain cells to rewire themselves. Such plasticity is essential to learning and memory.
In mice, the "brake" for the gene encoding MHC class I appears to be released twice: during early development and again in old age. Interestingly, late in life, the gene's neural expression occurs primarily in the hippocampus and other areas of the brain involved in learning and memory.
"MHC class I neurons may also play a role in age-related neurodegenerative diseases, such as Alzheimer's and Parkinson's,"says Shatz. "It may mistakenly signal the immune system to attack brain cells, just as it triggers a similar attack on the joints in cases of rheumatoid arthritis."
More recently, Shatz and her team have reported that neurons also express an immune system protein called paired-immunoglobulin-like receptor-B (PirB), which, over time, gradually inhibits brain plasticity. Mice that lack PirB exhibit greater synaptic plasticity as they age -- a finding that suggests that reducing PirB might help reestablish the connections among neurons damaged by spinal cord injury, stroke, or other trauma.
Together, these studies indicate that immune molecules perform important functions in the brain, including how much or how quickly our brain changes in response to new experiences. Researchers at the Karolinska Institute in Stockholm, Sweden, have found that removal of synapses from damaged neurons after a motor nerve injury, a process known as "synaptic stripping," is much stronger in mice who lack functioning MHC class I molecules. They also found that such mice are less likely to experience a regeneration of their motor neurons and that their glial cells react differently to the damaged neurons than do those of mice with functioning MHC class I molecules. "These results provide a surprising link between neuroscience and immunology," says Staffan Cullheim, MD, PhD. They also mark the first time a family of molecules has been linked directly to how the cell body of a neuron reacts after its axon -- the long projection that conducts electrical signals away from the cell's body -- has been injured.
In earlier studies, Cullheim and other scientists had reported that MHC class I molecules can be found in particularly high levels among motor neurons in the brain stem and the spinal cord, especially after the neurons have been damaged. In his most recent study, Cullheim found that the presence of MHC class I helps retain certain inhibitory synapses on the surface of injured motor neurons, thus reducing the likelihood that the neurons will fire a nerve impulse, or action potential, to neighboring cells.
MHC class I also has an effect on the action of glial cells, which in turn may influence neurons in various ways. Although microglia, the "immune cells" of the central nervous system, responded more weakly in the absence of MHC class I molecules, other glial cells, known as astrocytes, responded more vigorously. If -- and how -- these different responses are linked with synaptic stripping is not yet known.
"The consequences of the effects of MHC class I is still not clear," says Cullheim, "but it may be linked with the the ability of motor neurons to produce new axons. Mice with peripheral nerve lesions in their hind limbs exhibit less axonal bridging on those lesions when their MCH class I function is impaired." High levels of MHC class I, on the other hand, may pose a danger to neurons in the same way as is seen for other cell types -- during viral infection, for example. These high levels may even be involved in the development of neurodegenerative diseases. Research has shown that motor neurons involved in ALS and dopaminergic neurons involved in Parkinson's disease express among the largest amounts of MHC class I molecules in the nervous system. At the University of California, San Diego, Lisa Boulanger, PhD, and her colleagues have found that changes in the levels of specific immune molecules, members of the MHC class I family, are sufficient to cause cellular and behavioral symptoms of autism and schizophrenia in mice.
One set of preliminary studies from Boulanger's laboratory suggests that normal levels of MHC class I are needed for proper neuronal signaling by the neurotransmitter glutamate. The disruption of the glutamate signaling system is a hallmark of schizophrenia. It's also been recently characterized in patients with autism. In a second line of research, Boulanger has found that changes in MHC class I levels cause a striking disruption of the ability to "tune out" irrelevant sensory information, as measured by a neurological phenomenon known as prepulse inhibition, or PPI. Scientists have long known that PPI is impaired in people with schizophrenia, and recent studies suggest that it's also impaired in people with autism.
"We found in our current study that mice with altered levels of MHC class I share both abnormal glutamate signaling and this deficit in PPI," Boulanger says. "These results are exciting because they may provide clues to understanding the puzzle of why immune abnormalities are frequent among patients with autism and schizophrenia and their close relatives." Boulanger and her colleagues are currently investigating whether MHC class I molecules are altered in people with autism and schizophrenia. They are also using animal models to determine how immune signaling may affect the earliest events in fetal brain
development. "Human data show that in genetically predisposed individuals, a maternal viral infection during pregnancy increases the chance of the child developing either autism or schizophrenia later in life," says Boulanger. "Recent research in animal models suggests that it's not the infection itself, but rather an unknown, shared feature of the immune response to a variety of infectious agents that disrupts fetal brain development and leads to impairments in PPI."
A leading candidate for this mysterious immune trigger is the release of cellular signals called cytokines, which are produced during infection and injury. Cytokine levels are altered in the fetal brain following a maternal infection -- and in the brains of people with autism. Cytokines can increase the levels of MHC class I molecules in many types of cells, including neurons. "We're now trying to determine if changes in MHC class I molecules are the necessary link between maternal infections and abnormal fetal brain development," says Boulanger.
An experimental treatment called anti-NOGO-A immunotherapy has been found to improve performance on a test of cognitive ability after stroke in aged rats, according to a new study from a team of researchers led by Gwendolyn Kartje, MD, PhD, at Loyola University and the Edward Hines VA Hospital in Chicago. This finding may one day lead to more effective treatments for the millions of people worldwide who survive a stroke each year and for the millions of others suffering from Alzheimer's disease and other memory disorders. Anti-NOGO-A immunotherapy blocks the NOGO-A protein, a molecule found in the brain. The precise role of this protein is unknown, but it appears to inhibit aberrant growth. When the brain becomes damaged, however, this inhibitory function turns harmful, preventing injured cells from regenerating and repairing themselves. It also prevents uninjured cells from changing to help with the recovery.
In earlier studies, Kartje and her colleagues showed that anti-NOGO-A immunotherapy led to the recovery of forepaw and arm movement after induced stroke in aged rats. The new study found that the therapy also improved cognitive recovery when testing performance on a spatial memory task. "This suggests that the NOGO-A protein limits the recovery of memory after stroke and that by blocking the protein, more recovery may occur," Kartje says. Her laboratory next plans to look for structural changes in the brain that underlie the recovery process.
Adapted from materials provided by Society For Neuroscience.
newsday.com/news/health/ny-hsvacc125457394nov12,0,2245400.story
Newsday.com Report: Vaccine booster shots may be unnecessary BY DELTHIA RICKS
delthia.ricks@newsday.com
November 12, 2007
Vaccines have drawn an intense spotlight in recent years, and a study published last week raised a new question in the debate: Do Americans overvaccinate?
Scientists writing in the New England Journal of Medicine found that immunity lasts far longer than previously believed, suggesting that fewer booster shots may be warranted in adults. Still other doctors are wondering whether new vaccine approaches would better aid children.
At least one doctor would like to see childhood vaccinations spread out over a longer period of time.
Dr. Mark Slifka, an associate scientist with the Vaccine and Gene Therapy Institute in Oregon, wanted to know how long immunity lasts after vaccination or infection. He and his colleagues went into the study with a lot of strong hypotheses and "expected to see long-lived immunity following a viral infection and relatively short-lived immunity after vaccination." Those notions, Slifka and his team said, are the reasoning for booster shots.
To his surprise, the research revealed that the immunity the body marshals after vaccination with tetanus and diphtheria lasted far longer than scientists had once believed. Immunity that arose after certain viral infections, Slifka and collaborators discovered, were essentially maintained for life. Although it is important for the country to abide by vaccination as a vital public health tool, Slifka reported in the journal, it also is important to understand that boosters are not always necessary. "We also need to mention that overvaccinating the population poses no health or safety concerns," he said, adding "it may just be unnecessary under certain circumstances."
Dr. Len Horovitz, a pulmonary physician at Lenox Hill Hospital in Manhattan, said while the concept of overvaccination may sound radical and new, doctors have had the power for years to test a person's immunity after initial vaccination. Horovitz says he always tests students who come to see him prior to their first year of college. If they need a booster, he gives it. "It is possible to prevent this phenomenon," Horovitz said, referring to overvaccination, "by testing for antibodies." These immune-system proteins develop in the aftermath of vaccination. Antibodies are stimulated in the presence of a key protein called an antigen, a protein introduced by vaccination or infection.
The body "remembers" antigens through highly specialized, all-knowing constituents of the immune system: B cells, whose role is never to forget. When that memory fails, it can be reactivated with a booster shot. "To determine whether an MMR booster is needed," Horovitz said of the mumps-measles-rubella shot, "antibody testing for each antigen can be done so that an unnecessary vaccine is not administered.
"A lot of doctors do not test to see if a patient is still immune. When kids go to college, the university wants a kid to get a booster. It's very possible that a booster isn't needed - and you can test to get an answer. But there are a lot of doctors who'll say, 'Let's just give the kid a booster.'" Dr. Robert W. Sears, a vaccine expert and author of "The Vaccine Book," said parents of young children also use the term "overvaccination," but in a different way. They want to know whether vaccinations can be spread out to avoid a child's receiving so many shots at once.
"This is the single most important topic that I am most passionate about," Sears said. "Parents are concerned that simultaneous vaccines given to babies at an early age may be overwhelming to the infants' systems."
Just as Slifka sees no need for unnecessary boosters, Sears says it is possible to spread out vaccinations for children and still provide them with the same level of vital immunity to communicable diseases. "Vaccination is definitely important," Sears said. "Vaccines have played a tremendous role in eliminating or at least limiting certain diseases in our population."
But he adds that spreading out the shots is far less traumatic and does not compromise the benefit of immunization.
Copyright © 2007, Newsday Inc.
Public release date: 24-Oct-2007
http://www.eurekalert.org/pub_releases/2007-10/nsij-sda102307.php
Contact: Jamie Talan
jtalan@nshs.edu
516-562-1232
North Shore-Long Island Jewish (LIJ) Health System
Scientists discover a direct route from the brain to the immune system – It used to be dogma that the brain was shut away from the actions of the immune system, shielded from the outside forces of nature. But that’s not how it is at all. In fact, thanks to the scientific detective work of Kevin Tracey, MD, it turns out that the brain talks directly to the immune system, sending commands that control the body’s inflammatory response to infection and autoimmune diseases. Understanding the intimate relationship is leading to a novel way to treat diseases triggered by a dangerous inflammatory response.
Dr. Tracey, director and chief executive of The Feinstein Institute for Medical Research, will be giving the 2007 Stetten Lecture on Wednesday, Oct. 24, at the National Institutes of Health in Bethesda, MD. His talk – Physiology and Immunology of the Cholinergic Anti-inflammatory Pathway – will highlight the discoveries made in his laboratory and the clinical trials underway to test the theory that stimulation of the vagus nerve could block a rogue inflammatory response and treat a number of diseases, including life-threatening sepsis.
With this new understanding of the vagus nerve’s role in regulating inflammation, scientists believe that they can tap into the body’s natural healing defenses and calm the sepsis storm before it wipes out its victims. Each year, 750,000 people in the United States develop severe sepsis, and 215,000 will die no matter how hard doctors fight to save them. Sepsis is triggered by the body’s own overpowering immune response to a systemic infection, and hospitals are the battlegrounds for these potentially lethal conditions.
The vagus nerve is located in the brainstem and snakes down from the brain to the heart and on through to the abdomen. Dr. Tracey and others are now studying ways of altering the brain’s response or targeting the immune system itself as a way to control diseases.
Dr. Tracey is a neurosurgeon who came into research through the back door of the operating room. More than two decades ago, he was treating a young girl whose body had been accidentally scorched by boiling water and she was fighting for her life to overcome sepsis. She didn’t make it. Dr. Tracey headed into the laboratory to figure out why the body makes its own cells that can do fatal damage. Dr. Tracey discovered that the vagus nerve speaks directly to the immune system through a neurochemical called acetylcholine. And stimulating the vagus nerve sent commands to the immune system to stop pumping out toxic inflammatory markers. “This was so surprising to us,” said Dr. Tracey, who immediately saw the potential to use vagus stimulation as a way to shut off abnormal immune system responses. He calls this network “the inflammatory reflex.”
Research is now underway to see whether tweaking the brain's acetylcholine system could be a natural way to control the inflammatory response. Inflammation is key to many diseases - from autoimmune conditions like Crohn's disease and rheumatoid arthritis to Alzheimer's, where scientists have identified a strong inflammatory component.
Dr. Tracey has presented his work to the Dalai Lama, who has shown a great interest in the neurosciences and the mind-body connection. He has also written a book called “Fatal Sequence,” about the double-edge sword of the immune system.
About The Feinstein Institute for Medical Research
Headquartered in Manhasset, NY, The Feinstein Institute for Medical Research is home to international scientific leaders in Parkinson's disease, Alzheimer’s disease, psychiatric disorders, rheumatoid arthritis, lupus, sepsis, inflammatory bowel disease, diabetes, human genetics, leukemia, lymphoma, neuroimmunology, and medicinal chemistry. The Feinstein Institute, part of the North Shore-LIJ Health System, ranks in the top 6th percentile of all National Institutes of Health grants awarded to research centers. Feinstein researchers are developing new drugs and drug targets, and producing results where science meets the patient. For more information, please visit www.FeinsteinInstitute.org or http://feinsteininstitute.typepad.com/feinsteinweblog/
http://sciencenow.sciencemag.org/cgi/content/full/2008/521/4
Vaccine Booster's Secret Revealed
By Martin Enserink
ScienceNOW Daily News
21 May 2008
For decades, scientists have known that they can make vaccines much more efficacious by adding aluminum compounds, but they never knew why. Now, a study reveals how, on a molecular level, these helpers spur the production of antibodies. The finding may help researchers develop better vaccines.
Many vaccines contain adjuvants, nonspecific agents that help jolt the immune system into action. "Alum," a term referring broadly to aluminum hydroxide and several aluminum salts, has this effect, as was accidentally discovered in the 1920s. It has been widely used in human vaccines since the 1950s, and it's still the only adjuvant allowed in the United States. "But we didn't really have a clue about how it worked," says immunologist Harm HogenEsch of Purdue University's School of Veterinary Medicine in West Lafayette, Indiana. The dominant theory held that alum particles bind the antigen--the vaccine's main ingredient--on their surfaces, presenting them more slowly to the immune system and thus ensuring a more thorough response.
But the situation is more complicated than that. Last year, HogenEsch's team and a group led by Fabio Re at the University of Tennessee Health Science Center in Memphis showed that in macrophages--white blood cells that gobble up pathogens and cellular detritus--alum triggers the production of interleukin 1β and interleukin 18, two key signaling molecules, or cytokines, known to stimulate the production of antibodies. Researchers knew that this duo is often released after the activation of so-called NOD-like receptors. "So then the race was on," says Re, to pinpoint which NOD-like receptor was involved.
That race was won by a team led by Richard Flavell of Yale University. In this week's issue of Nature, Flavell's group reports that aluminum adjuvants trigger a NOD-like receptor called the Nalp3 inflammasome--an intracellular protein structure that plays a key role in immune activation. When the group injected mice lacking Nalp3 with an alum-boosted vaccine, they produced almost no antibodies; but a vaccine with another adjuvant called Freund's resulted in the usual, vigorous immune response. Re says he will publish the same result in a paper accepted by the Journal of Immunology, which also shows that two other adjuvants--QuilA and chitosan--work in the same way.
The Nalp3 inflammasome is known to be activated by compounds of microbial origin and also by molecules that appear when cells die, such as uric acid. So researchers think that Nalp3 is like a "danger sensor," says Yale immunologist Stephanie Eisenbarth, the first author on the Nature paper. Alum-containing vaccines may simply "hijack" that response.
Knowing how alum works its magic may help researchers design more specific adjuvants that are more effective or have fewer side effects, HogenEsch says. Alum, for instance, is known to kill muscle cells when injected into muscles, as many vaccines are.
Yale Researchers Describe How Vaccine Adjuvant Jump-Starts Immune System
New Haven, Conn. - Yale University researchers have determined how a key component of many vaccines activates an immune system response, a finding that opens up promising new avenues of research on better ways to prevent infections. A team of scientists led by Stephanie C. Eisenbarth and Richard A. Flavell of the departments of immunobiology and laboratory medicine at the Yale School of Medicine describe one way aluminum hydroxide th a key adjuvant used in many of the world's vaccines th helps fight off pathogens in a paper published Wednesday in the online edition of the journal Nature.
(Media-Newswire.com) - New Haven, Conn. — Yale University researchers have determined how a key component of many vaccines activates an immune system response, a finding that opens up promising new avenues of research on better ways to prevent infections.
A team of scientists led by Stephanie C. Eisenbarth and Richard A. Flavell of the departments of immunobiology and laboratory medicine at the Yale School of Medicine describe one way aluminum hydroxide – a key adjuvant used in many of the world’s vaccines – helps fight off pathogens in a paper published Wednesday in the online edition of the journal Nature.
Yale University scientists helped spur a revolution in immunology a decade ago by describing the key role played by Toll-like receptors, or TLRs, in triggering inflammatory responses. TLRs are a key receptor in the evolutionarily older and more generalized innate immune system that senses the presence of foreign invaders. TLRs must be activated before the younger adaptive immune system, - which can respond to specific pathogens and has long-term memory - can begin to fight infections in people.
However, scientists have found that the aluminum hydroxide, or alum, used in vaccines does not require that TLRs be activated to trigger an immune response, and the molecular mechanisms that explain its efficacy remained a mystery.
Eisenbarth and Flavell found that a crucial player in the process is a part of another weapon in the immune system’s arsenal called Nod-like receptors, specifically a protein complex called the Nalp3 inflammasome that is located within cells. They found that alum adjuvant activated the Nalp3 inflammasome, which is also triggered when cells come under stress. They also showed that when Nalp3 inflammasome was removed from cells they failed to produce cytokines known as interleukins, part of the immune system response usually triggered by the adjuvant. Also, antibody and T Cell responses were reduced in mice lacking parts of the inflammasome.
For vaccinologists, the paper is important because it describes at least one molecular basis for how an adjuvant like alum activates the immune system. Researchers hope to harness that knowledge to find new ways to use adjuvants to bolster immune system responses.
“As a physician, that is the most important thing. We need to know how these adjuvants actually work.’’ Eisenbarth said. (um yeah duh)
But as a researcher, Eisenbarth said, she is also fascinated by the role Nod-like receptors like the Nalp3 inflammasome might play in the fundamental activation of the arm of the immune system that mediates long-term protection against pathogens. “The paper also adds a new aspect to one of the most exciting fields in immunology – how the innate, or ancient immune system, needs to be activated before the more sophisticated adaptive immune system can do its work,’’ she said.
Other Yale researchers involved in the study are Oscar R. Colegio of the Departments of Dermatology and Immunobiology, William O’Connor Jr, of the Department of Immunology and Fayyaz S. Sutterwala, now of the University of Iowa.
http://media-newswire.com/release_1067044.html
Sci. & Tech.
Scientists discover how common vaccine booster works
In an online paper in the journal Nature, Yale University researchers funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, explain how a common ingredient in many vaccines stimulates and interacts with the immune system to help provide protection against infectious diseases.
According to Eurekalert, the news service of the American Association for the Advancement of Science, vaccines must possess not only the bacterial or viral components that serve as targets of protective immune responses, but also ingredients to kick start those immune responses. In many vaccines, the bacterial or viral components themselves have this capability. For other vaccines, the immune system requires an added boost. Adjuvants are those substances added to a vaccine to help stimulate the immune system and make the vaccine more effective.
Currently the only vaccine adjuvants licensed for general use in the United States are aluminum hydroxide/phosphate formulations, known as alum. Although alum has been used to boost the immune responses to vaccines for decades, no one has known how it worked.
In this paper, the Yale team, led by Richard Flavell, M.D., Ph.D., and Stephanie Eisenbarth, M.D., Ph.D., examined the immune system pathway and cell receptors used by alum. Many microbial compounds function as adjuvants by stimulating Toll-like receptors. These receptors identify microbial invaders and alert the body to the presence of a disease-causing agent, or pathogen. Alum, however, does not stimulate Toll-like receptors. The Yale team found that alum stimulates clusters of proteins called inflammasomes, found inside certain cells. Inflammasomes respond to stresses such as infection or injury by releasing immune cell signaling proteins called cytokines. Inflammasomes are a component of the innate immune system that operates in parallel with, but separate from, Toll-like receptors, also part of the innate immune system.
To make this determination, Dr. Eisenbarth and her coworkers used mice that had been genetically engineered to be deficient in various components of a specific type of inflammasome, characterized by the presence of the protein termed Nalp3. The team demonstrated that an immune response did not occur in those animals with the deficient Nalp3 inflammasomes, despite the inclusion of alum, while it did occur in normal mice. The team’s findings provide the first convincing evidence that the Nalp3 inflammasome forms the basis for alum’s adjuvant action.
According to the study authors, several unanswered questions remain regarding how activation of this pathway controls a highly specific and long-lasting immune response generated by a vaccine. But this new information on the molecules that alum uses to activate the innate immune system should provide the keys to better understanding adjuvant function and should facilitate the design of new vaccine adjuvants.
http://www.hindu.com/thehindu/holnus/008200805231024.htm
http://www.sciencedaily.com/releases/2008/05/080529141357.htm
Rett Syndrome Gene Is Full Of Surprises
ScienceDaily (Jun. 1, 2008) — A study funded by the National Institutes of Health (NIH) has transformed scientists' understanding of Rett syndrome, a genetic disorder that causes autistic behavior and other disabling symptoms. Until now, scientists thought that the gene behind Rett syndrome was an "off" switch, or repressor, for other genes. But the new study, published today in Science*, shows that it is an "on" switch for a startlingly large number of genes.
Rett syndrome is caused by a deficiency of the MECP2 gene. It occursalmost exclusively in girls, robbing them of language, cognitive and fine motor skills around the time they are learning to walk. Having extra copies of MECP2 can also cause Rett-like symptoms. By manipulating the number of copies of the ME CP2 gene in mice, the authors of the new study found that it controls thousands of other genes, suppressing some, but activating most. The research was funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), both part of NIH.
"This study simultaneously upends prevailing ideas about the disease process in MECP2-related disorders, and hints at new therapeutic strategies," says NIH Director Elias Zerhouni, M.D. Rett syndrome occurs predominantly in girls because the MECP2 gene is located on the X chromosome. In boys, who have only one X compared to girls' two, a deficiency of MECP2 tends to cause death during infancy. Girls with Rett syndrome tend to develop normally until about one year of age, and then regress in their language, cognitive and motor skills. They lose the words they have learned, as well as their skilled hand movements, which become replaced by repetitive wringing and clapping. Other common features include seizures, stunted growth and small brain size, mood disturbances, and sleep problems.
Duplications of MECP2 have been linked to another syndrome, which can cause Rett-like symptoms, and sometimes severe mental retardation, in boys. MECP2's dual roles in gene repression and activation were "a total surprise," says the lead author of the new study, Huda Zoghbi, M.D., a professor at Baylor College of Medicine in Houston and an investigator of the Howard Hughes Medical Institute. Dr. Zoghbi led the team that first linked MECP2 deficiencies to Rett syndrome in
1999, also an NIH-funded effort. Many lines of evidence pointed to the MeCP2 protein as a gene repressor, and that is how experts in the field, including Dr. Zoghbi, have defined its function for the past 10 years.
Dr. Zoghbi did not intend to question that definition. She was interested in comparing Rett syndrome and MECP2 duplication syndrome, and in adding to the list of the few genes known to be regulated by MECP2. Toward that end, she and her team analyzed gene activity patterns in the brains of mice with a MECP2 deficiency and in mice with a MECP2 duplication (MECP2+). Previous studies had revealed only subtle differences between the brains of normal and MECP2-mutant mice, but
those studies measured gene activity throughout the brain. Dr. Zoghbi's group focused on a brain region called the hypothalamus, which is known to produce hormones that influence growth, mood, and the sleep-wake cycle -- all of which typically become derailed in Rett syndrome.
Their analysis revealed nearly 2600 genes that are misregulated in both mouse models, with opposite patterns. The activity of about 2200 genes dropped in MECP2-deficient mice and spiked in MECP2+ mice, indicating that MECP2 is an activator for those genes. About 400 genes showed the reverse pattern, indicating that MECP2 is a repressor for those genes. In other experiments, the researchers confirmed that the MeCP2 protein binds directly to several of the target genes. They also found evidence that MeCP2 collaborates with another protein known to serve as a gene activator. Among the genes activated by MeCP2, the researchers found many that encode neuropeptides, proteins that are secreted by nerve cells.
All of these results raise a number of challenges and opportunities for future research, Dr. Zoghbi says. Researchers could design effective therapies for Rett syndrome and MECP2 duplication syndrome by aiming at MeCP2's target genes, but first they would have to know which target genes are most relevant to neurological function. Also, given that the two disorders have opposite gene activity profiles, they might not respond to the same therapies. The ideal therapy would aim closer to MECP2 itself, Dr. Zoghbi says. "We know that the MeCP2 protein is important for orchestrating gene expression in neurons," Dr Zoghbi says. "To treat the disease, we may need to find a way to re-orchestrate gene expression. The challenge is to identify the immediate lieutenants of MeCP2, and co-opt them to take over when MeCP2 is not working."
Journal reference:
Maria Chahrour, Sung Yun Jung, Chad Shaw, Xiaobo Zhou, Stephen T. C. Wong, Jun Qin, and Huda Y. Zoghbi. MeCP2, a Key Contributor to Neurological Disease, Activates and Represses Transcription. Science, 2008; 320 (5880): 1224 DOI: 10.1126/science.1153252 Adapted from materials provided by NIH/National Institute of Neurological Disorders and Stroke.
New Insights Into How Brain Responds To Viral Infection
ScienceDaily (Apr. 1, 2009) � Scientists at Columbia University
Mailman School of Public Health have discovered that astrocytes, supportive cells in the brain that are not derived from an immune cell lineage, respond to a molecule that mimics a viral infection using cellular machinery similar to that used by classical immune cells in the blood.
While scientists have been aware of the capacity of astrocytes to trigger an innate immune response when encountering a foreign agent, this work provides a new understanding of the complex mechanisms responsible for induction and regulation of inflammation in the brain and has significant implications for both the diagnosis and treatment of brain infections.
The study is published as the cover article in the April 2009 issue of The FASEB Journal.
In the course of trying to contain and neutralize a virus that has breached the protective barrier of the central nervous system, immune mediators secreted by astrocytes may injure other cells and tissues in the vicinity and cause additional life-threatening inflammation.
By defining the nature of inflammatory responses by brain astrocytes, this study has implications for both the diagnosis of chronic infections of the central nervous system, as well as the treatment of acute and chronic brain infections. Viral infections of the brain are associated with extremely high morbidity and mortality; in most cases, the specific microbial cause is unknown. Even when a viral cause is clear, the specific antiviral tools at our disposal remain limited. This work provides a means for implementation of a more general therapeutic approach to viral brain infections that may be effective across a wide range of viruses, or even where a virus is suspected but the offending agent cannot be identified.
http://www.sciencedaily.com/releases/2009/03/090331133341.htm
There are a number of diseases that this work can impact in terms of diagnosis and treatment: viral encephalitis; brain disorders associated with congenital viral infections; and neurological or neurodevelopmental disorders suspected of having an immune or inflammatory trigger, such as schizophrenia and autism. There also may be broader implications for the treatment of a wide range of immune-mediated neurologic diseases, such as multiple sclerosis and Parkinson's disease.
The microbial organ in the gut as a driver of homeostasis and disease
Mark Lyte
http://www.medical-hypotheses.com/article/PIIS030698770900704X/abstract?rss=yes
Received 12 October 2009; accepted 14 October 2009. published online 09 November 2009.
Corrected Proof
Summary
Based on the ability of bacteria to both recognize and synthesize neuroendocrine hormones, it is hypothesized that microbes within the intestinal tract comprise a community that interfaces with the mammalian nervous system that innervates the gastrointestinal tract to form a microbial organ. Given the evolutionary context in which the central nervous system is an outgrowth of the more primitive enteric nervous system and the time in which microbes have colonized the mammalian intestinal tract, it is further hypothesized that this microbial organ enters into a symbiotic relationship with its mammalian host to influence both homeostasis (aspects such as behavior) and susceptibility to disease. Contained within the overall hypothesis are three main thematic elements: the species composition of the microbial organ influences host homeostasis and disease susceptibility; the host’s nervous system influences the species composition of the microbial organ and the microbial organ itself possesses its own nervous system. Elucidation of the mechanisms by which this evolutionary symbiosis occurs would dramatically alter current medical thought by providing a biological basis for linking these two disparate organ systems and provide a new paradigm with which to understand and design new therapeutic approaches for a range of clinical diseases.
Department of Pharmacy Practice, School of Pharmacy, Texas Tech University Health Sciences Center, 3601 4th Street, MS8162, Lubbock, TX 79430, USA
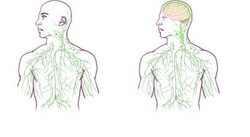
Public Release: 1-Jun-2015
Missing link found between brain, immune system -- with major disease implications Implications profound for neurological diseases from autism to Alzheimer's to multiple sclerosis
University of Virginia Health System
IMAGE: Maps of the lymphatic system: old (left) and updated to reflect UVA's discovery. view more
Credit: University of Virginia Health System
"Instead of asking, 'How do we study the immune response of the brain?' 'Why do multiple sclerosis patients have the immune attacks?' now we can approach this mechanistically. Because the brain is like every other tissue connected to the peripheral immune system through meningeal lymphatic vessels," said Jonathan Kipnis, PhD, professor in the UVA Department of Neuroscience and director of UVA's Center for Brain Immunology and Glia (BIG). "It changes entirely the way we perceive the neuro-immune interaction. We always perceived it before as something esoteric that can't be studied. But now we can ask mechanistic questions."
"We believe that for every neurological disease that has an immune component to it, these vessels may play a major role," Kipnis said. "Hard to imagine that these vessels would not be involved in a [neurological] disease with an immune component."
New Discovery in Human Body
Kevin Lee, PhD, chairman of the UVA Department of Neuroscience, described his reaction to the discovery by Kipnis' lab: "The first time these guys showed me the basic result, I just said one sentence: 'They'll have to change the textbooks.' There has never been a lymphatic system for the central nervous system, and it was very clear from that first singular observation - and they've done many studies since then to bolster the finding - that it will fundamentally change the way people look at the central nervous system's relationship with the immune system."
Even Kipnis was skeptical initially. "I really did not believe there are structures in the body that we are not aware of. I thought the body was mapped," he said. "I thought that these discoveries ended somewhere around the middle of the last century. But apparently they have not."
'Very Well Hidden'
The discovery was made possible by the work of Antoine Louveau, PhD, a postdoctoral fellow in Kipnis' lab. The vessels were detected after Louveau developed a method to mount a mouse's meninges - the membranes covering the brain - on a single slide so that they could be examined as a whole. "It was fairly easy, actually," he said. "There was one trick: We fixed the meninges within the skullcap, so that the tissue is secured in its physiological condition, and then we dissected it. If we had done it the other way around, it wouldn't have worked."
After noticing vessel-like patterns in the distribution of immune cells on his slides, he tested for lymphatic vessels and there they were. The impossible existed. The soft-spoken Louveau recalled the moment: "I called Jony [Kipnis] to the microscope and I said, 'I think we have something.'"
As to how the brain's lymphatic vessels managed to escape notice all this time, Kipnis described them as "very well hidden" and noted that they follow a major blood vessel down into the sinuses, an area difficult to image. "It's so close to the blood vessel, you just miss it," he said. "If you don't know what you're after, you just miss it."
"Live imaging of these vessels was crucial to demonstrate their function, and it would not be possible without collaboration with Tajie Harris," Kipnis noted. Harris, a PhD, is an assistant professor of neuroscience and a member of the BIG center. Kipnis also saluted the "phenomenal" surgical skills of Igor Smirnov, a research associate in the Kipnis lab whose work was critical to the imaging success of the study.
Alzheimer's, Autism, MS and Beyond
The unexpected presence of the lymphatic vessels raises a tremendous number of questions that now need answers, both about the workings of the brain and the diseases that plague it. For example, take Alzheimer's disease. "In Alzheimer's, there are accumulations of big protein chunks in the brain," Kipnis said. "We think they may be accumulating in the brain because they're not being efficiently removed by these vessels." He noted that the vessels look different with age, so the role they play in aging is another avenue to explore. And there's an enormous array of other neurological diseases, from autism to multiple sclerosis, that must be reconsidered in light of the presence of something science insisted did not exist.
Published in Nature
The findings have been published online by the prestigious journal Nature and will appear in a forthcoming print edition. The article was authored by Louveau, Smirnov, Timothy J. Keyes, Jacob D. Eccles, Sherin J. Rouhani, J. David Peske, Noel C. Derecki, David Castle, James W. Mandell, Lee, Harris and Kipnis.
###
The study was funded by National Institutes of Health grants R01AG034113 and R01NS061973. Louveau was a fellow of Fondation pour la Recherche Medicale.
Missing link found between brain, immune system -- with major disease implications Implications profound for neurological diseases from autism to Alzheimer's to multiple sclerosis
University of Virginia Health System
IMAGE: Maps of the lymphatic system: old (left) and updated to reflect UVA's discovery. view more
Credit: University of Virginia Health System
- Vessels directly connecting brain, lymphatic system exist despite decades of doctrine that they don't
- Finding may have substantial implications for major neurological diseases
- Game-changing discovery opens new areas of research, transforms existing ones
- Major gap in understanding of the human body revealed
- 'They'll have to change the textbooks'
"Instead of asking, 'How do we study the immune response of the brain?' 'Why do multiple sclerosis patients have the immune attacks?' now we can approach this mechanistically. Because the brain is like every other tissue connected to the peripheral immune system through meningeal lymphatic vessels," said Jonathan Kipnis, PhD, professor in the UVA Department of Neuroscience and director of UVA's Center for Brain Immunology and Glia (BIG). "It changes entirely the way we perceive the neuro-immune interaction. We always perceived it before as something esoteric that can't be studied. But now we can ask mechanistic questions."
"We believe that for every neurological disease that has an immune component to it, these vessels may play a major role," Kipnis said. "Hard to imagine that these vessels would not be involved in a [neurological] disease with an immune component."
New Discovery in Human Body
Kevin Lee, PhD, chairman of the UVA Department of Neuroscience, described his reaction to the discovery by Kipnis' lab: "The first time these guys showed me the basic result, I just said one sentence: 'They'll have to change the textbooks.' There has never been a lymphatic system for the central nervous system, and it was very clear from that first singular observation - and they've done many studies since then to bolster the finding - that it will fundamentally change the way people look at the central nervous system's relationship with the immune system."
Even Kipnis was skeptical initially. "I really did not believe there are structures in the body that we are not aware of. I thought the body was mapped," he said. "I thought that these discoveries ended somewhere around the middle of the last century. But apparently they have not."
'Very Well Hidden'
The discovery was made possible by the work of Antoine Louveau, PhD, a postdoctoral fellow in Kipnis' lab. The vessels were detected after Louveau developed a method to mount a mouse's meninges - the membranes covering the brain - on a single slide so that they could be examined as a whole. "It was fairly easy, actually," he said. "There was one trick: We fixed the meninges within the skullcap, so that the tissue is secured in its physiological condition, and then we dissected it. If we had done it the other way around, it wouldn't have worked."
After noticing vessel-like patterns in the distribution of immune cells on his slides, he tested for lymphatic vessels and there they were. The impossible existed. The soft-spoken Louveau recalled the moment: "I called Jony [Kipnis] to the microscope and I said, 'I think we have something.'"
As to how the brain's lymphatic vessels managed to escape notice all this time, Kipnis described them as "very well hidden" and noted that they follow a major blood vessel down into the sinuses, an area difficult to image. "It's so close to the blood vessel, you just miss it," he said. "If you don't know what you're after, you just miss it."
"Live imaging of these vessels was crucial to demonstrate their function, and it would not be possible without collaboration with Tajie Harris," Kipnis noted. Harris, a PhD, is an assistant professor of neuroscience and a member of the BIG center. Kipnis also saluted the "phenomenal" surgical skills of Igor Smirnov, a research associate in the Kipnis lab whose work was critical to the imaging success of the study.
Alzheimer's, Autism, MS and Beyond
The unexpected presence of the lymphatic vessels raises a tremendous number of questions that now need answers, both about the workings of the brain and the diseases that plague it. For example, take Alzheimer's disease. "In Alzheimer's, there are accumulations of big protein chunks in the brain," Kipnis said. "We think they may be accumulating in the brain because they're not being efficiently removed by these vessels." He noted that the vessels look different with age, so the role they play in aging is another avenue to explore. And there's an enormous array of other neurological diseases, from autism to multiple sclerosis, that must be reconsidered in light of the presence of something science insisted did not exist.
Published in Nature
The findings have been published online by the prestigious journal Nature and will appear in a forthcoming print edition. The article was authored by Louveau, Smirnov, Timothy J. Keyes, Jacob D. Eccles, Sherin J. Rouhani, J. David Peske, Noel C. Derecki, David Castle, James W. Mandell, Lee, Harris and Kipnis.
###
The study was funded by National Institutes of Health grants R01AG034113 and R01NS061973. Louveau was a fellow of Fondation pour la Recherche Medicale.
