Report: Walgreens Gave Wrong Flu Vaccine to Children Station says Walgreens acknowledged the mistake but won't say how many of the wrong doses of flu vaccine were administered.
Posted by Todd Richissin (Editor) , February 28, 2014 at 10:05 AM Walgreens not saying how many doses of wrong vaccine were administered. (Walgreens has given adult-strength flu vaccines to children in Roswell and possibly a larger number in the Atlanta metro area, according to news reports.
Heidi Sullivan told Channel 2 News that her two children received a flu shot in December at the Walgreens on West Crossville Road in Roswell and that a representative from Walgreens called her to tell her about the mix-up.
“They stuck a needle in each of my kids’ arms and gave them a drug that they should be responsible for knowing how to use properly,” Sullivan told Channel 2’s Rachel Stockman.
She learned the flu shot her 13-year-old and 16-year-old received was Flucelvax, a drug only approved by the Food and Drug Administration for those 18 and older, WSB News reported.
In a statement emailed to Channel 2 Action News, Jim Crohn, a spokesperson for Walgreens said:
“As part of the regular monitoring of our vaccine program, we recently learned that a very small percentage of vaccinations may have been administered to patients outside of FDA-approved age ranges.
“As a precautionary measure, we have already contacted all affected patients, along with their physicians where such information was available, and also apologized for this occurrence. We will continue to take proactive steps to help ensure the highest levels of quality care for our program.”
“How many other people are affected by this because Walgreens is a huge company?” Sullivan said.
Walgreens would not tell the station how many children or what stores in the metro Atlanta area were affected by this apparent mistake.
It is not clear what the side effects are for children because the approved FDA tests were done on adults.
The most common side effect for adults includes headache, discomfort and fatigue, the station reported.
http://canton-ga.patch.com/groups/around-town/p/report-walgreens-gave-wrong-flu-vaccine-to-children
Posted by Todd Richissin (Editor) , February 28, 2014 at 10:05 AM Walgreens not saying how many doses of wrong vaccine were administered. (Walgreens has given adult-strength flu vaccines to children in Roswell and possibly a larger number in the Atlanta metro area, according to news reports.
Heidi Sullivan told Channel 2 News that her two children received a flu shot in December at the Walgreens on West Crossville Road in Roswell and that a representative from Walgreens called her to tell her about the mix-up.
“They stuck a needle in each of my kids’ arms and gave them a drug that they should be responsible for knowing how to use properly,” Sullivan told Channel 2’s Rachel Stockman.
She learned the flu shot her 13-year-old and 16-year-old received was Flucelvax, a drug only approved by the Food and Drug Administration for those 18 and older, WSB News reported.
In a statement emailed to Channel 2 Action News, Jim Crohn, a spokesperson for Walgreens said:
“As part of the regular monitoring of our vaccine program, we recently learned that a very small percentage of vaccinations may have been administered to patients outside of FDA-approved age ranges.
“As a precautionary measure, we have already contacted all affected patients, along with their physicians where such information was available, and also apologized for this occurrence. We will continue to take proactive steps to help ensure the highest levels of quality care for our program.”
“How many other people are affected by this because Walgreens is a huge company?” Sullivan said.
Walgreens would not tell the station how many children or what stores in the metro Atlanta area were affected by this apparent mistake.
It is not clear what the side effects are for children because the approved FDA tests were done on adults.
The most common side effect for adults includes headache, discomfort and fatigue, the station reported.
http://canton-ga.patch.com/groups/around-town/p/report-walgreens-gave-wrong-flu-vaccine-to-children
More than 20,000 need to get new shots after vaccines go bad
http://www.kgw.com/news-local/stories/kgw_072109_health_bad_vaccines.62371ba8.html
02:04 PM PDT on Tuesday, July 21, 2009
By DAVID KROUGH, kgw.com Staff
PORTLAND, Ore. – Thousands of patients are being asked to get re-vaccinated in cities across Oregon after some refrigerated dosages may have gone bad. The vaccines may have been stored at temperatures that render them ineffective, no one was at risk for any medical problems from the shots, officials said.
The Oregon health department said around 22,000 patients at three private clinics in Oregon would be notified.
The vaccines most affected were childhood shots against tetanus, diphtheria, pertussis, polio, measles, mumps, rubella, flu, hepatitis A & B, human papillomavirus, viral influenza and pneumococcal disease.
The vaccines had been stored and given out at the High Desert Medical Center in Burns, Providence Health & Services in Gateway, Milwaukie and Sunnyside clinics in the Portland area; Cannon Beach, Seaside and Warrenton clinics on the north coast; and Doctors Clinic, Jacksonville, Medford Family Practice and Shady Cove clinics in southern Oregon. The Corvallis Clinic, with approximately 14,000 patients who received vaccine from its clinics in Albany, Corvallis and Philomath.
There will be no charge to the patients.
More information can be found at Oregon Health Dept.
Wonder what this is about......http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Recalls/ucm172378.htm
Recall: Prevnar Pneumococcal 7-valent Conjugate Vaccine, Wyeth DATE RECALL INITIATED:
July 10, 2009
PRODUCT / LOT NUMBER / EXPIRATION DATE:
Prevnar pneumococcal 7-valent Conjugate Vaccine
(Diptheria CRM197Protein)
0.5 mL single dose pre-filled syring (10 per package)
NDC: 0005-1970-50 (10’s)/0005-1970-49 (Singles)
Lot Number: D50002
Expiration Date: February 28, 2011
MANUFACTURER:
Wyeth
Philadelphia, PA
REASON:
Wyeth is voluntarily recalling the above lot of Prevnar®, Pneumococcal 7-valent Conjugate Vaccine, single dose pre-filled syringes. During a routine physical reconciliation of Prevnar® pre-filled syringes, Wyeth determined that a portion of a bulk lot of pre-filled syringes, which was not intended for commercial use, was inadvertently packaged and distributed with commercial product under Lot D50002. The product distributed as Lot D50002 met Wyeth’s quality acceptance criteria. Although some of the units of Lot D50002 were not intended for the commercial market, Wyeth performed a medical assessment and has concluded that the affected syringes present no health or safety risk to patients and that there is no need to revaccinate.
PULSE POLIO BUNGLE
UNTESTED VACCINE SURFACES IN POLIO OUTBREAK
A potent new vaccine introduced in Uttar Pradesh by the WHO has had no safety tests; the rash of new polio cases in the state may’ve been caused by the vaccine itself, reports Mihir Srivastava
Surrounded by mango groves, village Rahimabad is situated 10 kilometers off the Lucknow-Sitapur highway in the Khairabad block of Sitapur district. Rahimabad is in news for a dubious reason. A two-year-old girl of this village, Saniya, suffers from Type I polio despite being administered more than seven doses of the new polio monovalent vaccine (MOPVI), which is made specially for the Type I poliovirus. The vaccine was introduced in mid-2005 and tom-tommed as the final step in the eradication of polio from India. Before its introduction, a trivalent vaccine was in use that simultaneously targeted the three poliovirus strands found in India, Type I, II and III, by introducing into the body live viruses of all the three strands to develop immunity.
Saniya’s is not the only case. There are 15 cases of Type I polio spread across Uttar Pradesh (There are also 41 cases of Type II polio which takes the total count to 56). While there has been no reported Type I case in the endemic Moradabad, the new cases have been reported from eastern and central Uttar Pradesh; so instead of just a region, cases of wild polio are being reported from all over Uttar Pradesh now.
Saniya’s mother, Noorjahen, is furious. “She is having polio drops ever since she was four days old. She has had over a dozen doses of the polio drops.
(And this is a mystery why she has polio? )
We came to know about her polio when she got a high fever. She could barely manage to stand, could not walk at all, after the fever. We took her to the local hospital where they did a stool test. We were later told that she has polio,” she recounts. “There must be some thing wrong with the polio drops if even after so many doses my child has contracted polio. The government should test medicines before they are used. Pata nahin bachchoo ko kya pila rahin hain!” (Don’t know what they are making my child drink), she adds.
Mistrust is not only rife among the patients’ families, it has also gripped the doctors and field operatives overseeing the vaccination project. Add to this the latest controversy about the MOPVI vaccine, introduced in India by the World Health Organisation (who), and the organisation’s National Polio Surveillance Project (npsp), and you get a sense of the callousness plaguing the polio campaign.
Pulse of the polio programme
Thursday October 18 2007 09:12 IST Farah Baria
http://newindpress.com/NewsItems.asp?ID=IE220071017225054&Title=Second+Article&rLink=0
Not long ago, a gentleman turned up at our door with a large box. “Madam,” he said gravely, “according to our records your children have not availed of the government’s Pulse Polio Programme yesterday. Please ensure that this is done now.”
Two days later, my daughter complained of a pain in the leg. “Could be vaccine-associated paralytic polio,” warned my doctor. Eventually that turned out to be a false alarm. But it left me with a vague sense of dread.
The disquiet returned when a fouryear- old Mumbai girl died of the virus recently. Alarmingly, the child’s records show she had received several doses of the vaccine this year. More alarmingly, the Pulse Polio Programme prescribes one dose every month, way in excess of the internationally prescribed standard of seven doses over a lifetime.
Government officials believe this unprecedented blitzkrieg will check the resurgence of our Ninja bug — 676 fresh cases in 2006, another 223 this year — and plug “coverage lapses” in the implementation of its aggressive Rs 2,000 crore Polio Eradication Initiative (PEI). The strategy: propaganda and door-todoor implementation.
At one level, this meticulous planning is impressive in a country where public health initiatives die early of official neglect. Meticulous it may be but it is not exactly democratic. Even if I am a layperson, as a parent I have some questions to ask. Is this battery of “supplementary” vaccinations safe for our children? Does the government have the authority to prescribe it in the absence of any long-term studies? And what about our right to be informed about the possible side-effects?
Incidentally, question three remains a moot point. Reason: medical literature maintains that the Oral Polio Vaccine (OPV) occasionally backfires because it contains a live virus which can mutate and become neuro-virulent, causing Vaccine Associated Paralytic Polio, also known as Acute Flaccid Paralysis (AFP). Despite the “negligible” risk, in 1997, there were 3,047 cases of AFP; in 2005, 26,000. Also, to be effective, the OPV must be continuously stored in temperatures below minus two degrees centigrade. In a land of power cuts, this is virtually impossible.
Interestingly, many western countries have reverted to an older, more stable, Injectable Polio Vaccine (IPV) which uses a dead virus that does not require cold storage, and carries no known risk. Recently, an article in Lancet warned that the OPV is giving rise to a new strain called Vaccine Derived Polio Virus, and recommended the use of IPV.
This is controversial, especially since the OPV seems tailor-made for India. It is about six times cheaper than IPV, easier to administer, and has a “herd immunisation” effect, protecting both child and community. But the government needs to weigh the merits and demerits of both through democratic debate, not high-handed policy. Surely as parents we have the right to make an informed choice on matters that concern our children’s health. And we would like to know why we are using the OPV for our children after the West spurned it?
And how we square these questions with the WHO declaring last year that India is “actively exporting polio” to other countries. It even threatened to issue a travel advisory requiring Indians travelling overseas to provide proof of vaccination. Subsequently, the Union health minister called polio a “national shame” and vowed to atone. Again, while it is understandable to maintain that such advisories are avoidable, the government must act when warnings are based on demonstrable facts.
Disease prevention must remain essentially, indeed wholly, a medical exercise, informed by the best medical evidence and choices. Which is to say, the Polio Eradication Initiative cannot and must not become an exercise in diplomacy.
Let’s aim for some perspective. Polio is a dreadful bug which must be eradicated. But we should ask ourselves if we are going about it the right way — and for the right reasons. We may even perhaps need to pause, revaluate, and rethink
Common Children's Vaccine Recalled
By MIKE STOBBE and LINDA A. JOHNSON – 2 hours ago
ATLANTA (AP) — More than a million doses of a common vaccine given to babies as young as 2 months were being recalled Wednesday because of contamination risks, but the top U.S. health official said it was not a health threat.
The recall is for 1.2 million doses of the vaccine for Hib, which protects against meningitis, pneumonia and other serious infections, and a combination vaccine for Hib and hepatitis B. The vaccine is recommended for all children under 5 and is usually given in a three-shot series, starting at 2 months old.
Drug maker Merck & Co., which announced the recall after testing this week showed a sterility problem in a Pennsylvania factory, said concerned parents should contact their child's doctor.
"The potential for contamination of any individual vaccine is low," said Merck spokeswoman Kelley Dougherty.
Dr. Julie Gerberding, head of the Centers for Disease Control and Prevention, echoed that in a news conference.
"This is not a health threat in the short run, but it is an inconvenience," she said.
Merck produces about half of the nation's annual supply of 14 million doses of Hib vaccine.
Barbara Kuter, executive director of pediatric medical affairs for Merck, told The Associated Press that because of the contamination, the company's production line has been shut down for at least nine months.
"Manufacture of vaccines is pretty complicated, and we have to basically make some changes in the process," then get approval from the Food and Drug Administration before resuming production and shipments, Kuter said. Merck hopes to restart production in the fourth quarter of 2008, she said.
"It's likely that there's going to be a shortage of this product," Kuter said, adding that the impact on the public is unclear because the other company making the vaccine in the U.S., Sanofi Pasteur, may be able to produce more.
Health officials said they already are talking about prioritizing shots for American Indian and Alaska Native children, who are considered at higher risk for Hib-caused illnesses, said Dr. Anne Schuchat, director of the CDC's National Center for Immunization and Respiratory Diseases.
Health officials said they did not know how many of the 1.2 million doses were administered to children.
The recalled doses are considered potent, so children who got vaccine from the recalled lots will not have to be revaccinated, Schuchat said.
Parents will probably be concerned, CDC officials acknowledged. Should the vaccine later prove contaminated, health officials believe most children will experience, at worst, a skin irritation around the vaccination site. Problems could be worse for children with compromised immune systems.
Such problems would have appeared within one week of the vaccination, Schuchat said, adding that there have been no reports suggesting vaccine contamination so far.
The contamination involved unspecified equipment used in making the vaccine, which involves taking concentrated Hib virus, diluting it and combining it with other agents. Kuter said that during a routine evaluation of Merck's West Point, Pa., vaccine plant, a sterility test determined that the equipment was contaminated with a bacteria called Bacillus cereus, or B. cereus.
It is a spore-making microorganism commonly associated with food poisoning and has caused diarrhea and vomiting in people who eat contaminated foods.
"It's one of the most common organisms" around, Kuter said.
The recall is likely to heighten a debate over childhood vaccines and their safety and whether too many are required. Some parents are distrustful and suspect some vaccines of being linked to autism, although scientific studies have not shown such a connection.
This week, New Jersey took a controversial step toward becoming the first state to require flu shots for preschoolers after a health advisory board backed new vaccine requirements over opposition from parents.
Merck, based in Whitehouse Station, N.J., is one of the few drug makers that produces a significant number of vaccines.
While the company took a black eye with its September 2004 withdrawal of the painkiller Vioxx due to increased risk of heart attacks and strokes, the company has been performing well recently. On Tuesday, it gave an upbeat assessment in its annual briefing for analysts.
Five weeks ago, Merck reached a deal to settle up to 50,000 Vioxx lawsuits for $4.85 billion, an amount expected to save the company millions in trial costs.
Its stock price has more than recovered from its post-Vioxx slump, a two-year-old restructuring plan is going well and profits are up. For example, Merck posted a 62 percent increase in its third-quarter profit as revenues jumped by double digits.
The company also has had an impressive seven new products approved for U.S. sale in the last two years, including three vaccines: RotaTeq, to prevent an intestinal virus that is the top cause of early childhood diarrhea; Zostavax to prevent shingles, and Gardasil, to block the virus that causes cervical cancer.
Merck shares fell 68 cents Wednesday to close at $59.72 before the recall announcement. The shares fell 12 cents in after-hours trading.
AP Medical Writer Mike Stobbe reported from Atlanta and AP Business Writer Linda A. Johnson reported from Trenton, N.J.
Chinese Parents Take "Bad Vaccines" Case To Court
By Tan Ee Lyn, Reuters Health
http://tinyurl.com/2fa7co
HONG KONG (Reuters) - Three children who suffered severe brain damage after being vaccinated against Japanese encephalitis had their case heard in a Chinese court on Thursday, casting the spotlight once again on the safety of China's drugs and food. Ordinary citizens suing powerful state companies are rare in China and this case is especially sensitive as it calls into question the standards and safety of Chinese medicines. "Our chances of winning are zero because our opponents are mighty, but we won't back down," said Yu Tongan, father of one of the three. "As victims we have to stand up not only for ourselves but to tell the
world such things are happening to us and many, many other children." Passed by mosquitoes, Japanese encephalitis can result in paralysis, seizures, coma and death. It is endemic in most parts of Asia, and countries such as China, India, South Korea, Japan, Taiwan and Thailand control the disease with vaccines. At the start of the two-day hearing, Tang Jingling, a lawyer representing the children and their families, told the court in Jiangmen city, in southern Guangdong province, how Liang Jiayi, a lively two-year-old, ran a high fever after she was given a vaccine shot in a government clinic in August 2003. She fell into a coma four days later and when she came to, she was paralysed and has remained in a vegetative state. The other two children, Tan Jieyi and Yu Ronghui, were vaccinated in Jiangmen in March 2005. Now 12 and 14 respectively, they can walk but are mentally retarded and have been refused places in school.
JUST BAD LUCK?
Defendants named in the lawsuit were vaccine manufacturer Chengdu Institute of Biological Products, and the Centers for Disease Control and Prevention in Xinhui district and Jiangmen. "Treatments for all three children are ongoing and they hope to get compensation ... of around 1 million yuan ($132,200) each ... for
their medical fees and disabilities," Tang told Reuters later. Parents of the affected children had petitioned authorities in Beijing, southern Guangzhou city and even Hong Kong, but were arrested when they mounted a protest in Beijing's Tiananmen Square last year. But now a court has agreed to hear their case, weeks after a senior Chinese official was executed for taking bribes and approving unsafe drugs. The parents say they have asked for an explanation of what happened to their children, only to be told by officials that it was bad luck. China's Health Ministry told Reuters last year that there were no problems with the vaccines. Rare complications happen with any kind of vaccine, especially when recipients have poor immunity or genetic defects. But Tang said is this case there was a fairly large cluster of seven children in Jiangmen who were all injected in March 2005 - raising questions over its quality. Liang, now 6, and her parents were not in court but in Beijing, where she was recuperating from recent brain surgery. "She was getting very frequent seizures and was constantly foaming in he mouth. We had to raise money to bring her here for surgery," her father, Liang Yongli, told Reuters by telephone. "We want the state authorities that are responsible for this to cure her."
OSAKA: Out-of-date ingredient used in vaccine
06/29/2007
THE ASAHI SHIMBUN
A foundation affiliated with Osaka University used out-of-date ingredients in a measles-rubella vaccine that was shipped for 530,000 people from March, sources said.
The vaccine, produced in 2004 by the Research Foundation for Microbial Diseases of Osaka University, contained Australian cattle blood serum whose quality guarantee date had expired in 2002.
The batch of vaccine passed government tests and was shipped.
Although the Ministry of Health, Labor and Welfare said the out-of-date ingredients will not affect the safety of the vaccine, critics say the manufacturing process of medical products should be made more transparent to ensure product safety.(IHT/Asahi: June 29,2007)
http://www.kgw.com/news-local/stories/kgw_072109_health_bad_vaccines.62371ba8.html
02:04 PM PDT on Tuesday, July 21, 2009
By DAVID KROUGH, kgw.com Staff
PORTLAND, Ore. – Thousands of patients are being asked to get re-vaccinated in cities across Oregon after some refrigerated dosages may have gone bad. The vaccines may have been stored at temperatures that render them ineffective, no one was at risk for any medical problems from the shots, officials said.
The Oregon health department said around 22,000 patients at three private clinics in Oregon would be notified.
The vaccines most affected were childhood shots against tetanus, diphtheria, pertussis, polio, measles, mumps, rubella, flu, hepatitis A & B, human papillomavirus, viral influenza and pneumococcal disease.
The vaccines had been stored and given out at the High Desert Medical Center in Burns, Providence Health & Services in Gateway, Milwaukie and Sunnyside clinics in the Portland area; Cannon Beach, Seaside and Warrenton clinics on the north coast; and Doctors Clinic, Jacksonville, Medford Family Practice and Shady Cove clinics in southern Oregon. The Corvallis Clinic, with approximately 14,000 patients who received vaccine from its clinics in Albany, Corvallis and Philomath.
There will be no charge to the patients.
More information can be found at Oregon Health Dept.
Wonder what this is about......http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Recalls/ucm172378.htm
Recall: Prevnar Pneumococcal 7-valent Conjugate Vaccine, Wyeth DATE RECALL INITIATED:
July 10, 2009
PRODUCT / LOT NUMBER / EXPIRATION DATE:
Prevnar pneumococcal 7-valent Conjugate Vaccine
(Diptheria CRM197Protein)
0.5 mL single dose pre-filled syring (10 per package)
NDC: 0005-1970-50 (10’s)/0005-1970-49 (Singles)
Lot Number: D50002
Expiration Date: February 28, 2011
MANUFACTURER:
Wyeth
Philadelphia, PA
REASON:
Wyeth is voluntarily recalling the above lot of Prevnar®, Pneumococcal 7-valent Conjugate Vaccine, single dose pre-filled syringes. During a routine physical reconciliation of Prevnar® pre-filled syringes, Wyeth determined that a portion of a bulk lot of pre-filled syringes, which was not intended for commercial use, was inadvertently packaged and distributed with commercial product under Lot D50002. The product distributed as Lot D50002 met Wyeth’s quality acceptance criteria. Although some of the units of Lot D50002 were not intended for the commercial market, Wyeth performed a medical assessment and has concluded that the affected syringes present no health or safety risk to patients and that there is no need to revaccinate.
PULSE POLIO BUNGLE
UNTESTED VACCINE SURFACES IN POLIO OUTBREAK
A potent new vaccine introduced in Uttar Pradesh by the WHO has had no safety tests; the rash of new polio cases in the state may’ve been caused by the vaccine itself, reports Mihir Srivastava
Surrounded by mango groves, village Rahimabad is situated 10 kilometers off the Lucknow-Sitapur highway in the Khairabad block of Sitapur district. Rahimabad is in news for a dubious reason. A two-year-old girl of this village, Saniya, suffers from Type I polio despite being administered more than seven doses of the new polio monovalent vaccine (MOPVI), which is made specially for the Type I poliovirus. The vaccine was introduced in mid-2005 and tom-tommed as the final step in the eradication of polio from India. Before its introduction, a trivalent vaccine was in use that simultaneously targeted the three poliovirus strands found in India, Type I, II and III, by introducing into the body live viruses of all the three strands to develop immunity.
Saniya’s is not the only case. There are 15 cases of Type I polio spread across Uttar Pradesh (There are also 41 cases of Type II polio which takes the total count to 56). While there has been no reported Type I case in the endemic Moradabad, the new cases have been reported from eastern and central Uttar Pradesh; so instead of just a region, cases of wild polio are being reported from all over Uttar Pradesh now.
Saniya’s mother, Noorjahen, is furious. “She is having polio drops ever since she was four days old. She has had over a dozen doses of the polio drops.
(And this is a mystery why she has polio? )
We came to know about her polio when she got a high fever. She could barely manage to stand, could not walk at all, after the fever. We took her to the local hospital where they did a stool test. We were later told that she has polio,” she recounts. “There must be some thing wrong with the polio drops if even after so many doses my child has contracted polio. The government should test medicines before they are used. Pata nahin bachchoo ko kya pila rahin hain!” (Don’t know what they are making my child drink), she adds.
Mistrust is not only rife among the patients’ families, it has also gripped the doctors and field operatives overseeing the vaccination project. Add to this the latest controversy about the MOPVI vaccine, introduced in India by the World Health Organisation (who), and the organisation’s National Polio Surveillance Project (npsp), and you get a sense of the callousness plaguing the polio campaign.
Pulse of the polio programme
Thursday October 18 2007 09:12 IST Farah Baria
http://newindpress.com/NewsItems.asp?ID=IE220071017225054&Title=Second+Article&rLink=0
Not long ago, a gentleman turned up at our door with a large box. “Madam,” he said gravely, “according to our records your children have not availed of the government’s Pulse Polio Programme yesterday. Please ensure that this is done now.”
Two days later, my daughter complained of a pain in the leg. “Could be vaccine-associated paralytic polio,” warned my doctor. Eventually that turned out to be a false alarm. But it left me with a vague sense of dread.
The disquiet returned when a fouryear- old Mumbai girl died of the virus recently. Alarmingly, the child’s records show she had received several doses of the vaccine this year. More alarmingly, the Pulse Polio Programme prescribes one dose every month, way in excess of the internationally prescribed standard of seven doses over a lifetime.
Government officials believe this unprecedented blitzkrieg will check the resurgence of our Ninja bug — 676 fresh cases in 2006, another 223 this year — and plug “coverage lapses” in the implementation of its aggressive Rs 2,000 crore Polio Eradication Initiative (PEI). The strategy: propaganda and door-todoor implementation.
At one level, this meticulous planning is impressive in a country where public health initiatives die early of official neglect. Meticulous it may be but it is not exactly democratic. Even if I am a layperson, as a parent I have some questions to ask. Is this battery of “supplementary” vaccinations safe for our children? Does the government have the authority to prescribe it in the absence of any long-term studies? And what about our right to be informed about the possible side-effects?
Incidentally, question three remains a moot point. Reason: medical literature maintains that the Oral Polio Vaccine (OPV) occasionally backfires because it contains a live virus which can mutate and become neuro-virulent, causing Vaccine Associated Paralytic Polio, also known as Acute Flaccid Paralysis (AFP). Despite the “negligible” risk, in 1997, there were 3,047 cases of AFP; in 2005, 26,000. Also, to be effective, the OPV must be continuously stored in temperatures below minus two degrees centigrade. In a land of power cuts, this is virtually impossible.
Interestingly, many western countries have reverted to an older, more stable, Injectable Polio Vaccine (IPV) which uses a dead virus that does not require cold storage, and carries no known risk. Recently, an article in Lancet warned that the OPV is giving rise to a new strain called Vaccine Derived Polio Virus, and recommended the use of IPV.
This is controversial, especially since the OPV seems tailor-made for India. It is about six times cheaper than IPV, easier to administer, and has a “herd immunisation” effect, protecting both child and community. But the government needs to weigh the merits and demerits of both through democratic debate, not high-handed policy. Surely as parents we have the right to make an informed choice on matters that concern our children’s health. And we would like to know why we are using the OPV for our children after the West spurned it?
And how we square these questions with the WHO declaring last year that India is “actively exporting polio” to other countries. It even threatened to issue a travel advisory requiring Indians travelling overseas to provide proof of vaccination. Subsequently, the Union health minister called polio a “national shame” and vowed to atone. Again, while it is understandable to maintain that such advisories are avoidable, the government must act when warnings are based on demonstrable facts.
Disease prevention must remain essentially, indeed wholly, a medical exercise, informed by the best medical evidence and choices. Which is to say, the Polio Eradication Initiative cannot and must not become an exercise in diplomacy.
Let’s aim for some perspective. Polio is a dreadful bug which must be eradicated. But we should ask ourselves if we are going about it the right way — and for the right reasons. We may even perhaps need to pause, revaluate, and rethink
Common Children's Vaccine Recalled
By MIKE STOBBE and LINDA A. JOHNSON – 2 hours ago
ATLANTA (AP) — More than a million doses of a common vaccine given to babies as young as 2 months were being recalled Wednesday because of contamination risks, but the top U.S. health official said it was not a health threat.
The recall is for 1.2 million doses of the vaccine for Hib, which protects against meningitis, pneumonia and other serious infections, and a combination vaccine for Hib and hepatitis B. The vaccine is recommended for all children under 5 and is usually given in a three-shot series, starting at 2 months old.
Drug maker Merck & Co., which announced the recall after testing this week showed a sterility problem in a Pennsylvania factory, said concerned parents should contact their child's doctor.
"The potential for contamination of any individual vaccine is low," said Merck spokeswoman Kelley Dougherty.
Dr. Julie Gerberding, head of the Centers for Disease Control and Prevention, echoed that in a news conference.
"This is not a health threat in the short run, but it is an inconvenience," she said.
Merck produces about half of the nation's annual supply of 14 million doses of Hib vaccine.
Barbara Kuter, executive director of pediatric medical affairs for Merck, told The Associated Press that because of the contamination, the company's production line has been shut down for at least nine months.
"Manufacture of vaccines is pretty complicated, and we have to basically make some changes in the process," then get approval from the Food and Drug Administration before resuming production and shipments, Kuter said. Merck hopes to restart production in the fourth quarter of 2008, she said.
"It's likely that there's going to be a shortage of this product," Kuter said, adding that the impact on the public is unclear because the other company making the vaccine in the U.S., Sanofi Pasteur, may be able to produce more.
Health officials said they already are talking about prioritizing shots for American Indian and Alaska Native children, who are considered at higher risk for Hib-caused illnesses, said Dr. Anne Schuchat, director of the CDC's National Center for Immunization and Respiratory Diseases.
Health officials said they did not know how many of the 1.2 million doses were administered to children.
The recalled doses are considered potent, so children who got vaccine from the recalled lots will not have to be revaccinated, Schuchat said.
Parents will probably be concerned, CDC officials acknowledged. Should the vaccine later prove contaminated, health officials believe most children will experience, at worst, a skin irritation around the vaccination site. Problems could be worse for children with compromised immune systems.
Such problems would have appeared within one week of the vaccination, Schuchat said, adding that there have been no reports suggesting vaccine contamination so far.
The contamination involved unspecified equipment used in making the vaccine, which involves taking concentrated Hib virus, diluting it and combining it with other agents. Kuter said that during a routine evaluation of Merck's West Point, Pa., vaccine plant, a sterility test determined that the equipment was contaminated with a bacteria called Bacillus cereus, or B. cereus.
It is a spore-making microorganism commonly associated with food poisoning and has caused diarrhea and vomiting in people who eat contaminated foods.
"It's one of the most common organisms" around, Kuter said.
The recall is likely to heighten a debate over childhood vaccines and their safety and whether too many are required. Some parents are distrustful and suspect some vaccines of being linked to autism, although scientific studies have not shown such a connection.
This week, New Jersey took a controversial step toward becoming the first state to require flu shots for preschoolers after a health advisory board backed new vaccine requirements over opposition from parents.
Merck, based in Whitehouse Station, N.J., is one of the few drug makers that produces a significant number of vaccines.
While the company took a black eye with its September 2004 withdrawal of the painkiller Vioxx due to increased risk of heart attacks and strokes, the company has been performing well recently. On Tuesday, it gave an upbeat assessment in its annual briefing for analysts.
Five weeks ago, Merck reached a deal to settle up to 50,000 Vioxx lawsuits for $4.85 billion, an amount expected to save the company millions in trial costs.
Its stock price has more than recovered from its post-Vioxx slump, a two-year-old restructuring plan is going well and profits are up. For example, Merck posted a 62 percent increase in its third-quarter profit as revenues jumped by double digits.
The company also has had an impressive seven new products approved for U.S. sale in the last two years, including three vaccines: RotaTeq, to prevent an intestinal virus that is the top cause of early childhood diarrhea; Zostavax to prevent shingles, and Gardasil, to block the virus that causes cervical cancer.
Merck shares fell 68 cents Wednesday to close at $59.72 before the recall announcement. The shares fell 12 cents in after-hours trading.
AP Medical Writer Mike Stobbe reported from Atlanta and AP Business Writer Linda A. Johnson reported from Trenton, N.J.
Chinese Parents Take "Bad Vaccines" Case To Court
By Tan Ee Lyn, Reuters Health
http://tinyurl.com/2fa7co
HONG KONG (Reuters) - Three children who suffered severe brain damage after being vaccinated against Japanese encephalitis had their case heard in a Chinese court on Thursday, casting the spotlight once again on the safety of China's drugs and food. Ordinary citizens suing powerful state companies are rare in China and this case is especially sensitive as it calls into question the standards and safety of Chinese medicines. "Our chances of winning are zero because our opponents are mighty, but we won't back down," said Yu Tongan, father of one of the three. "As victims we have to stand up not only for ourselves but to tell the
world such things are happening to us and many, many other children." Passed by mosquitoes, Japanese encephalitis can result in paralysis, seizures, coma and death. It is endemic in most parts of Asia, and countries such as China, India, South Korea, Japan, Taiwan and Thailand control the disease with vaccines. At the start of the two-day hearing, Tang Jingling, a lawyer representing the children and their families, told the court in Jiangmen city, in southern Guangdong province, how Liang Jiayi, a lively two-year-old, ran a high fever after she was given a vaccine shot in a government clinic in August 2003. She fell into a coma four days later and when she came to, she was paralysed and has remained in a vegetative state. The other two children, Tan Jieyi and Yu Ronghui, were vaccinated in Jiangmen in March 2005. Now 12 and 14 respectively, they can walk but are mentally retarded and have been refused places in school.
JUST BAD LUCK?
Defendants named in the lawsuit were vaccine manufacturer Chengdu Institute of Biological Products, and the Centers for Disease Control and Prevention in Xinhui district and Jiangmen. "Treatments for all three children are ongoing and they hope to get compensation ... of around 1 million yuan ($132,200) each ... for
their medical fees and disabilities," Tang told Reuters later. Parents of the affected children had petitioned authorities in Beijing, southern Guangzhou city and even Hong Kong, but were arrested when they mounted a protest in Beijing's Tiananmen Square last year. But now a court has agreed to hear their case, weeks after a senior Chinese official was executed for taking bribes and approving unsafe drugs. The parents say they have asked for an explanation of what happened to their children, only to be told by officials that it was bad luck. China's Health Ministry told Reuters last year that there were no problems with the vaccines. Rare complications happen with any kind of vaccine, especially when recipients have poor immunity or genetic defects. But Tang said is this case there was a fairly large cluster of seven children in Jiangmen who were all injected in March 2005 - raising questions over its quality. Liang, now 6, and her parents were not in court but in Beijing, where she was recuperating from recent brain surgery. "She was getting very frequent seizures and was constantly foaming in he mouth. We had to raise money to bring her here for surgery," her father, Liang Yongli, told Reuters by telephone. "We want the state authorities that are responsible for this to cure her."
OSAKA: Out-of-date ingredient used in vaccine
06/29/2007
THE ASAHI SHIMBUN
A foundation affiliated with Osaka University used out-of-date ingredients in a measles-rubella vaccine that was shipped for 530,000 people from March, sources said.
The vaccine, produced in 2004 by the Research Foundation for Microbial Diseases of Osaka University, contained Australian cattle blood serum whose quality guarantee date had expired in 2002.
The batch of vaccine passed government tests and was shipped.
Although the Ministry of Health, Labor and Welfare said the out-of-date ingredients will not affect the safety of the vaccine, critics say the manufacturing process of medical products should be made more transparent to ensure product safety.(IHT/Asahi: June 29,2007)

Indonesia's Polio Vaccination Hampered By Rumors, Ignorance By MICHAEL CASEY
& ASSOCIATED PRESS
Published on 8/28/2005
Cikeusal, Indonesia— Holding her 2-year-old son, Sari listens intently in a ramshackle health clinic as the medical staff assures her and other villagers about the safety of the vaccine being used to fight Indonesia's first polio outbreak in a decade.
But the impoverished mother of two remains unconvinced. She hints she will not participate in Tuesday's nationwide immunization campaign because of unfounded rumors that a neighbor's child contracted polio after being given the oral vaccine earlier this year.
“I'm afraid. Maybe my boy will get paralyzed,” said Sari, who was among 62 percent of parents in her village who refused to get their children vaccinated in June during a regional campaign on Java, the main island where most of the nation's 226 polio cases have occurred.
Such fears are threatening the biggest public health exercise ever mounted in Indonesia, whose rising caseload has the World Health Organization worried that the virus could spread throughout Southeast Asia.
Indonesian leaders are pulling out all stops to win over a public skeptical about the drive to vaccinate 24 million children under age 5 on Tuesday and then again on Sept. 27.
The two largest Muslim organizations in the world's most populous Islamic nation are endorsing vaccinations in TV ads, and busloads of soap opera stars and singers are making the rounds to promote a $24 million campaign comparable in preparation to a general election.
More than 750,000 vaccinators will be on hand Tuesday at 245,000 posts set up at health clinics, bus depots, rail stations and airports. The army and police will help deliver vaccine — by plane, boat, bicycle and foot — to some of Indonesia's 6,000 inhabited islands.
“The biggest challenge is public trust,” said UNICEF's Claire Hajaj, who works on the U.N. agency's global campaign to eradicate polio in the six countries where it remains endemic, as well as in Indonesia and 16 other nations that recently have been re-infected.
“The key is that community fears get addressed and they don't turn into widespread vaccine avoidance,” Hajaj said.
A 20-month-old diagnosed with polio in March was the country's first case since 1995. Authorities believe the child caught it from a migrant worker or tourist who was infected in Africa or the Middle East.
Polio spreads when unvaccinated people come into contact with the feces of those with the virus, often through contaminated water in places with poor hygiene or inadequate sewage systems. It attacks the nervous system in children under 5, causing paralysis, muscular atrophy and sometimes death, although only about one in 200 of those infected ever develops symptoms.
The potential for the virus to spread beyond Indonesia's 210 million people has prompted East Timor, the Philippines and Thailand to launch smaller vaccination campaigns.
“If this virus continues to spread, we are talking potentially hundreds more Indonesians becoming paralyzed,” said Arun Thapa, who is overseeing WHO's polio eradication campaign in Southeast Asia and visited Indonesia this past week.
Indonesia's polio outbreak first prompted authorities to vaccinate as many as 6.5 million children in Java province during two rounds earlier this year.
But officials missed 1 million children in the second round after parents were scared off by false media reports that three children died from taking vaccine or rumors that the vaccine violates Islamic law.
The rumors mirrored those that spread across the West African nation of Nigeria in 2003, where polio vaccinations were suspended for several months after radical Islamic preachers told parents they were dangerous and part of a U.S. plot against Muslims.
Islamic leaders in Indonesia sought to put such rumors to rest by issuing a fatwa saying the vaccine does not violate Muslim dietary law. But in provinces like West Java, which has 58 polio cases, that and other rumors persist.
Confusion also lingers among health workers over basic policies, such as whether sick children can be vaccinated. UNICEF says they can.
“Everything is going well, but we are still worried people won't take the vaccine,” said Dr. Agus Gusmara, who heads the campaign in Serang district. “We have still have bad memories of the last two rounds.”
© The Day Publishing Co., 2005
For home delivery, please call 1-866-846-9099
Asheville mother wants answers after 15-year-old son dies suddenly
http://www.citizen-times.com/cache/article/news/71621.shtml By Rebeccah Cantley-Falk, STAFF WRITER Dec. 1, 2004 2:22 p.m. ASHEVILLE - An Asheville mother is desperately seeking answers after her 15-year-old son died Tuesday shortly after receiving a vaccine for Hepatitis B.
Lori Watkins said her son, Kennie Ray Nelson Jr., collapsed after receiving the vaccine at Asheville Children's Medical Center. Nelson then went into convulsions and died around 11:30 a.m., Watkins said. Nelson was pronounced dead Tuesday at Mission Hospitals, spokeswoman Merrell Gregory said. Bonnie Smith, practice manager at Asheville Children's Medical Center, said she could not comment.
"We can't say anything at this point and time," she said.
Hepatitis B is a serious disease caused by a virus that attacks the liver. The virus can cause lifelong infection, cirrhosis (scarring) of the liver, liver cancer, liver failure and death.
Watkins had taken her son for a physical and to get his vaccinations updated in preparation for an outdoors camp he planned to attend. She was waiting Wednesday afternoon for a report from the Medical Examiner to find out what exactly happened to him.
"I was doing what I thought I was supposed to do for my son," Watkins said. "I was trying to protect him from something that could make him sick. And then, the vaccine itself ends up killing him?"
Check back at www.CITIZEN-TIMES.com for more breaking news updates. See Thursday's Citizen-Times newspaper for more on this story.
Families Raise Concern Over Mercury In Vaccines
Debate Continues Over Past Use Of Thimerosal
POSTED: 1:37 p.m. EST November 4, 2002
11:08 p.m. EST November 4, 2002 DURHAM COUNTY, N.C.
A growing contingent of parents believes a mercury-based preservative in those vaccines may have done more harm than good. In 1999, at the request of the Food and Drug Administration, drug companies agreed to begin removing a controversial preservative called thimerosal from vaccines. Some families believe the removal comes too late. Jackson Bono is a happy, curious 13-year-old challenged by a myriad of medical and developmental problems. Jackson has trouble speaking and focusing and works with a tutor.
"The toll it takes on a family is remarkable," said Scott Bono, Jackson's father. Like most parents, Scott and Laura Bono had their son vaccinated when he was a baby. They now blame his problems on thimerosal and its main ingredient, mercury.
"Little did we ever suspect that the very immunizations that were to protect him from childhood diseases were poisoning him with mercury," Scott Bono said. Thimerosal kills harmful bacteria and has been in vaccines for decades. In the early 1990s, the number of recommended childhood vaccines increased. Over the last decade the national autism rate has risen drastically. In North Carolina, the rate has more than quadrupled, according to the state Department of Public Instruction.
Some people see a connection. If you add up the amount of mercury in baby vaccines with thimerosal, the levels exceed those considered safe for adults by the FDA. The Bonos said Jackson was a normal, healthy baby until he received a bundle of vaccines when he was 16 months old. They said, soon after, he stopped talking and making eye contact. Jackson developed autistic tendencies, like spinning uncontrollably. He also suffered severe allergies, seizures and stomach trouble.
"It was a cruel tragedy that happened with our son," Laura Bono said. Dr. Samuel Katz, chairman emeritus of pediatrics at Duke, is considered one of the foremost authorities on vaccines in the country. He raises doubts that thimerosal ever hurt children. "Whenever we have a problem, we like to know whose fault is it. Unfortunately, vaccines have become an easy target," he said. Katz said, "The evidence to support these claims is lacking." However, in 1999, he recommended drug companies take thimerosal out of vaccines. A 2001 report from the National Institute of Medicine also concluded the evidence does not support the claims. Researchers conceded, "the hypothesis is biologically plausible."
"Given that its mercury and we know that mercury has no beneficial effects, my statement to the FDA was that there's really no reason to use something like thimerosal," said Michael Aschner, a Wake Forest University neurobiologist. Aschner has studied mercury for 20 years. Research from the University of Calgary backs up his work and found mercury can destroy brain cells. Aschner points out that the ethylmercury in thimerosal is different from the damaging methylmercury found in some fish. He feels the issue clearly deserves much more study.
"If you do it in a dish, ethylmercury does cause significant effects, toxic effects. There's no question about it," Ascher said. "But, again, what you have to be careful of is how you translate what you see in a dish into a human being." The biggest obstacle parents of special needs children face in making the thimerosal argument is the fact that millions of children, a vast majority, got the same vaccine and never got sick.
"Why is it that all people who smoke don't get cancer? The body reacts differently to different antagonists," Salisbury attorney Bill Graham said. Graham represents 40 families who believe thimerosal hurt their children. He believes evidence is mounting that federal regulators knew that thimerosal could be harmful long before drug companies felt pressure to remove it from vaccines. A study sanctioned by the Centers for Disease Control and Prevention shows infants immunized with thimerosal vaccines were 2.5 times more likely to develop neurological disorders, but it was never released. Instead, the study continued and the results changed. Graham questions why vaccines were never recalled.
"Do you think that thimerosal vaccines that are potentially harmful could still be out there? They could be. They could be on the shelf right now," Graham said. "I really think the thimerosal issue has become a feeding frenzy. It's like the sharks with blood in the water," Katz said. The Bonos said they do not want blood. They want families like theirs to be heard for Jackson's sake, and others like him. "He's lost his childhood and he may not ever be what he should have been," Laura Bono said. Parents like the Bonos can file claims with the National Vaccine Injury Compensation Program. Because of the debate over thimerosal, the federal government has put all the claims on hold until further studies are completed. There was no recall of thimerosal vaccines, so it is possible some could still be on shelves. Anyone with concerns should talk to their child's pediatrician and ask for thimerosal-free vaccines. Both sides of the debate stress the importance of immunizing children.
Reporter: Cullen Browder
Photographer: Gil Hollingsworth
OnLine Producer: Michelle Singer
http://timesofindia.indiatimes.com/cms.dll/html/comp/articleshow?artid=28006270
Hep-B vaccine drive a farce, claims PEEM
TIMES NEWS NETWORK [ WEDNESDAY, NOVEMBER 13, 2002 02:00:00 AM ]
HYDERABAD: All children suffering from the side-effects of the hepatitis-B vaccination campaign in the state must be compensated by Bill Gates, founder of the Bill and Melinda Gates Foundation. The hepatitis-B campaign in the state is a farce and unnecessary. This was the contention of People for Economical and Effective Medicare (PEEM) chairman Dr P V R Bhaskar Rao at a press conference here on Tuesday.
It was well-known that there are adverse effects of the vaccine. The US Congress, having accepted this, has included the vaccine in the National Vaccine Injury Compensation Programme in August 1998, Dr Rao said. Further, he said in the United States, authorities no longer advocate that all newborns be vaccinated against hepatitis-B unless they are born to infected mothers.
Despite this, the state government has no compensation policy for victims suffering from adverse effects of the vaccine, no registry of the children the vaccine has been administered on, no studies about the effectiveness of the vaccine in the state and no information education and communication (IEC) activities carried out, Dr Rao said.
PEEM representatives also appealed to all parents of children who have received the vaccine to claim for a damage of Rs 25 lakh if the child has any side-effects from the Gates foundation. According to the National Vaccination Information Centre (NVIC) report, the protective effect of the vaccine was still unknown, PEEM vice chairman Dr K Venugopal said. Further, the Federal Drug Authority (FDA) has reported that between 1994 and 1998, a total of 23,000 cases of adverse effects due to the vaccine. The PEEM said Bill Gates, his foundation and the state government were using children of the state as guinea pigs.
WASHINGTON (AP) -- A vaccine that cleared toxic deposits from the brains of laboratory mice also doubled the risk of stroke in the animals, according to a study that may offer a new warning sign about a promising therapy for Alzheimer's disease.
The study, appearing Friday in the journal Science, is the first to detect in animals serious side effects of a proposed vaccine that other research has suggested will halt the progression of Alzheimer's disease. A similar vaccine was tested briefly in humans. Researchers have been intensively studying the idea that the brain-destroying disease could be controlled by removing deposits of a toxic substance called amyloid beta that accumulates in the brains of Alzheimer's disease patients.
While researchers are uncertain if amyloid beta is the cause of Alzheimer's or the result of another mind-destroying process, some earlier animal studies found that a vaccine that prompts the body to remove amyloid beta was able to halt the disease and even restore some brain function. The promising results led an Irish pharmaceutical firm, Elan Corp., to test an amyloid beta vaccine on 360 human patients, but the clinical trial was suspended early this year after 15 patients developed inflammation of tissues in the brain. Elan has since said it would no longer test the original vaccine, but will continue to monitor patients who received it.
Unexpected side effects
Now the new study in Science suggests that a vaccine against amyloid beta may have other problems -- a significant increase of bleeding in the brain. Swiss, German and American researchers used a lab mouse strain that had been genetically manipulated to develop the major symptoms of Alzheimer's disease, including the formation of amyloid beta. The mice were injected with a vaccine that caused their bodies to make antibodies against amyloid beta. After five months, the researchers said there was a 23 percent reduction of the amyloid beta in the test mice compared with animals that did not receive the vaccine.
However, the test mice also had twice the number of cerebral hemorrhages, or bleeding in the brain, compared with mice that did not get the vaccine. Additionally, the researchers found six major blood clots among the immunized mice, versus only one among the control mice. The findings, said co-author Dr. Paul M. Mathews of New York University School of Medicine, suggest that scientists still lack a clear appreciation of the possible side effects of Alzheimer's vaccine therapy. "Up to this point, all of the animal studies have been very promising," said Mathews. "This is the first study to show any serious side effects in mice." Back to the drawing board Mathews said the mouse strain used in his study is a closer mimic to the way Alzheimer's appears in the human brain than animals used in earlier studies. He said the mice had deposits of amyloid beta on blood vessels, a very common characteristic of human patients.
Finding this new side effect, he said, suggests that the human trials of Alzheimer's vaccine "were premature." "We need to move back into animals and sort this out," said Mathews. "We need to develop antibodies (vaccines) that don't cause this problem. Otherwise, I don't think this (type of therapy) will work in humans." Bill Thies, vice president for medical and scientific affairs of the Alzheimer's Association, said the new study "is an interesting paper and something that we should look at carefully."
But he said Alzheimer's vaccine therapy remains promising and research should be pursued vigorously. "We are continuing to unravel some of the nuances of it," said Thies. "Whether it ends up being a useful therapy or not is still an open question, but it has enough promise that a lot of people are still working on it."
http://www.cnn.com/2002/HEALTH/conditions
/11/15/alzheimer.study.ap/index.ht=
West Nile Vaccine- Adverse Reaction
Horse Owner Speaks Out
Before I speak, let me preface this article to make it clearly understood, this information is not a blame or vendetta towards anything or anyone. It also needs to be unmistakably understood that I am not for or against vaccinating horses. My sole intent and/or purpose is to educate and inform the horse owner regarding concerns of their horse(s) health in an effort to assist and/or prevent the tragic misfortune that happened to our gelding, Dartanian. He was one of the principal characters in the book, "The Spiritual Life of Horses." His love, integrity, courage, loyalty and quite often humor, will live on in our hearts through his inspiration and valor.
In mid August we were suggested to vaccinate against West Nile Virus, I simply was not well-educated on the vaccine nor the virus or knew the right questions to ask at the time. With the first shot, three of our horses reacted. Our pregnant mare, our gelding and Dr. Donald Warren's stallion, a boarder, all had the same re-action, their front legs swelled. Within 2 days, our gelding Dartanian foundered, shortly there after he died! These are adverse reactions to a vaccine. The reactions our horses experienced are not soley limited to what we saw. Other adverse reactions stated by Fort Dodge's safety study are: systemic reactions, colic, diarrhea, fever, swellings.
The West Nile Vaccine is new. According to Dr. Charles L. McDaniel from USDA, "this is what is called a conditional vaccine. New vaccines such as this, are granted a one year "conditional" trial period. If safety and efficacy are established, a renewal license for a second year may be granted." The West Nile Vaccine license has been renewed for another year. He also stated "when a new vaccine is released it is sold and provided only through veterinarians for one reason, because the manufacturer needs to monitor the field response." (**more information from Dr. Charles McDaniel regarding conditional licensing)
If any reaction occurs then it is the responsibility of the practicing veterinarian to record it and report it to the manufacturer immediately in case there is a problem with that serial batch of vaccine. In the case of the West Nile Vaccine, the manufacturer is Fort Dodge. I spoke with Dr. Tuttle at Fort Dodge regarding our situation and also explained what I was dealing with afterward. Dr. Tuttle, listened intently to what I described, documented my report and said to me, "Lynette, please accept my greatest apologies for what you have gone through, I am a practicing veterinarian, this information is not falling on deaf ears, I will investigate this immediately."
He also stated, "we urge and encourage people to contact us immediately and report adverse reactions to our vaccines. We subsequently report it to the USDA and investigate the serial numbers of the batches that were being used to see if the batch has been contaminated. If people don't report problems then we don't know." Dr. Tuttle also stated to me, " if there is the slightest indication that the vaccine caused a problem then it needs to be reported to us without delay so that we can investigate and research that batch." If your horse(s) have had a reaction to this vaccine, Dr. Charlie McDaniel, USDA and Dr. Tuttle from Fort Dodge are asking you to contact them and report it. The following is their contact information:
Dr. Charlie McDaniel, USDA,
Email: charles.1.mcdaniel@aphis.usda.gov
Tel# 1-515-232-5785 ext. 146
Also www.usp.org/vprp.htm this is a website where you document what happened. Once the information is received it is therefore reported to the manufacturer. The manufacturer then investigates and researches the problem. If a bad batch of vaccine has made it's way to the public it is then recalled. This is my understanding of recourse from both Dr. McDaniel and Dr. Tuttle.
Dr. Tuttle, Fort Dodge,
Email: info@equinewestnile.com Attn: West Nile Product Manager
Tel# 1-800-533-8536
Alzheimer's treatment makes mice brains bleed Immunization used in halted human trial may weaken blood vessels. 15 November 2002
JOHN WHITFIELD
Alzheimer's attacks blood vessels, as well as brain tissue.
© SPL
Immunizing mice against a condition akin to Alzheimer's disease makes their brains prone to bleeding, researchers have found1. This hints at why cerebral inflammation halted an experimental human vaccine trial early this year. The link between mouse and human symptoms is still unknown. But both probably stem from the effects of immunization on damaged blood vessels, says the study's leader, Mathias Jucker of the University of Basel, Switzerland. "These findings are pretty bad for the vaccine," comments neuroscientist Christian Haass of Ludwig Maximilians University, Munich, Germany. "The bleeding is terrible - it could be deadly."
The results suggest that immunization may be more suited to protecting healthy brains than curing diseased ones. Another possibility would be to screen patients for vulnerable blood vessels before vaccination. But other Alzheimer's experts believe that the different symptoms and side-effects in mice and humans make it impossible to connect this research with the trials. "It would be a giant leap to apply this to an Alzheimer's patient," says Roger Nitsch of the University of Zurich, Switzerland.
This is the first time that bleeding has been seen in any animal or human test of Alzheimer's therapies. "We've never seen anything like this - and we've looked hard for it," comments Dale Schenk, head of research at Elan Pharmaceuticals of South San Francisco, the company behind the trial vaccine. The mice that Elan use to study Alzheimer's do not have damaged blood vessels, Jucker counters - so one would not expect immunization to affect them in this way.
Despite the setback, Alzheimer's researchers are still optimistic about the prospects for immunization. Most agree that more results are needed from humans for us to truly understand immunization's effects. Follow-up studies and post mortems of the 375 people immunized in the aborted trial should still give us invaluable information. Beta test The brains of Alzheimer's sufferers contain deposits of a protein called amyloid beta. These are thought to relate to the brain damage and dementia that are symptomatic of the disease. Three years ago, researchers at Elan proposed that injecting amyloid beta into the blood triggers an immune response that fights the disease. No one is yet sure how this works.
Tests were spectacular in mice engineered to develop a form of Alzheimer's. Amyloid deposits shrank, and the animals' memories improved. The vaccine was made from a synthetic version of amyloid. But trials in Alzheimer's sufferers were halted in January, when some patients developed symptoms similar to meningitis and encephalitis. The new finding suggests how this may have come about.
It would be a giant leap to apply this to an Alzheimer's patient Roger Nitsch University of Zurich
Jucker and his colleagues injected elderly mice with antibodies against amyloid, rather than amyloid itself. Five months later, the mice had smaller deposits of the protein in their brains. But they also had many small haemorrhages in cerebral blood vessels. The mice - and most human Alzheimer's patients - have amyloid deposits in the brain's blood vessels, as well as its tissue. Clearing out the protein might weaken these vessels. Vaccinating people before they get Alzheimer's, or in the very early stages of the disease, might help, as amyloid would not have had time to build up, says Richard Harvey, research director of Britain's Alzheimer's Society.
But if immunized human patients bled, it is surprising that none of them had a stroke, Harvey adds. "I doubt that the bleeding is the whole story". Bleeding may be a second side-effect to put alongside inflammation, agrees neuroscientist Dave Morgan of the University of South Florida. <http://www.nature.com/nsu/slices/spacer_trans.gif
References
* Pfeifer, M. et al. Cerebral hemorrhage after passive anti-AB immunotherapy.
Science, 298, 1379, (2002). |Homepage|
© Nature News Service / Macmillan Magazines Ltd 2002
http://www.cm-life.com/vnews/display.v/ART/2002/10/30/3dbf7da3740a9
Meningitis vaccines recalled
By Amanda Cutler
Central Michigan Life
October 30, 2002
Aventis Pasteur issued a recall of four of its single-dose lots of Menomune vaccine, which protects against four strains of bacterial meningitis. Aventis Pasteur is an international researcher, developer, manufacturer and supplier of vaccines. Affected lots were issued after Jan. 2, 2001. University Health Services, which issues the vaccine, did not receive any of the affected lots, said Sarah Campbell, director of University Health Services.
“As a precaution, the company withdrew not only those four lots, but all of the single-dose vials and we did have some single-dose vials,” she said. The company recommends that anyone who received the vaccine from the recalled and withdrawn lots, and is planning to travel to a high-risk country should contact their health care provider to discuss re-vaccination. “They tested some of the vaccine to see if it was effective at the 6-month and 12-month point,” Campbell said. “In four single-dose lots, they discovered that at the 12-month point, it did not protect against one of the four strains of bacterial meningitis.”
Symptoms associated with serogroup A, the strain of bacteria not protected against, include severe headache, stiff neck, nausea and vomiting, fever, a rash and mental confusion. The disease progresses rapidly, Campbell said. Someone affected by the disease could experience symptoms in the morning and be near death in the evening. “There has only been one case of serogroup A meningitis in the United States in the last 10 years,” she said. “So, the people that would need to be concerned are people that were traveling to other parts of the world where they do have epidemics of the serogroup A meningitis.”
Other people who might be affected are those who work in a laboratory or industrial setting, dealing with the serogroup A bacteria, she said. “It’s fairly rare, but we encourage students to consider the vaccination, because of the fact that it is potentially fatal,” Campbell said. Even when it’s not fatal, there are sometimes very serious complications that include amputations, organ failure and brain damage, she said. “I think its important to note that the vaccine still does provide protection against serogroups C, Y and W-135,” Campbell said. “Those are the strains that have been occurring most commonly in outbreaks on college campuses.” University Health Services is still giving the vaccine with the multiple-dose vials, which provides full protection, she said. Students can contact University Health Services at 774-6599 or visit the Primary Care Suite in Foust Hall 104, if they have concerns.
http://www.wfsb.com/Global/story.asp?S=1025686
COLCHESTER -- State health experts are investigating an outbreak of chicken pox in Colchester. 65 students at the Jack Jackter Elementary School got chicken pox last year. It was one of the largest outbreaks in the state. The Centers for Disease Control is also involved in the investigation. That's because some of the students who got sick were vaccinated against chicken pox. The chicken pox vaccine has a 20-percent failure rate.
http://mdn.mainichi.co.jp/news/20021129p2a00m0dm006000c.html
Group outbreak of deadly measles confirmed
KITAIBARAKI, Ibaraki -- A group of junior high school pupils who fell ill earlier this year have been confirmed as Japan's first group outbreak of a potentially deadly measles virus, health officials said Friday. The 109 pupils from Kitaibaraki who fell ill from February to April were afflicted with a H-1 measles virus of a type that has struck widely in China and South Korea. Over 70 percent of the afflicted pupils had been immunized against measles, yet still fell ill. "We need to look into what caused their immunity to weaken," a spokesman from the Ibaraki Prefectural Government's preventative medicine section said. Kitaibaraki Municipal Government officials said the prefectural government will examine why so many of the 109 pupils fell ill even though they had received shots to prevent them from picking up measles. Usually when measles breaks out in Japan, it is carried by D3 or D5 viruses. The differences between these and the H1 virus are not great, but enough for the H1 virus to counter some defense barriers formed by immunization. (Mainichi Shimbun, Nov. 29, 2002)
http://www.chinapost.com.tw/taiwan/detail.asp?ID=32734&GRP=B
Newborn baby killed,six others injured by mistaken
injection
2002/11/30
The China Post staff
One newborn baby was killed, and six others injured yesterday when a nurse at a Taipei County hospital mistakenly injected them with anesthetics that caused them to fall into comas, health officials said. The nurse at Tucheng's Peicheng Hospital was supposed to give the babies hepatitis B vaccinations, but mistakenly injected them with a muscle-relaxing drug, Atracurium, for patients due to undergo surgery, the Department of Health (DOH) officials said. The babies, who had stopped breathing as a result of the injection, were rushed separately to bigger hospitals in the city and county of Taipei for emergency treatment at about 9 a.m., but one of them was pronounced dead in the afternoon. The other six babies were reported to be in a stable condition, but doctors said they had yet to determine whether they had sustained brain damage due to a lack of oxygen.
"Something's wrong, hurry up," a Peicheng nurse, surnamed Liang, reported a panicking voice from within the baby room while she was not far from room teaching mothers how to take care of their new- born babies at about 800 a.m. Liang said before she realized what had happened, emergency crews were rushing the babies away to other hospitals. DOH Secretary-General Lai Chin-hsiang said prosecutors are now investigating the criminal and civil liabilities in the case. A report of the findings of the investigation will be submitted the medical oversight committee, and the hospital may be punished with a license revocation, Lai said. The 21-year-old nurse who committed the lethal mistake, Huang Ching-hui, was granted NT$250,000 bail after an interrogation by the Taipei District Prosecutors Office.
Peicheng director, Hsu Mu-chuan, admitted to an administrative error on the part of the hospital, partly blaming the new packaging of the hepatitis B vaccination for the mistake. Hsu said the vaccines, used to be stored in one-shot (1 cc) bottles, has recently repacked into 10-cc bottles, which he said were similar to those of the muscle-relaxing drug. "We deeply regret the mistake," said Hsu, adding the hospital would give a preliminary compensation of NT$100,000 to each of the victims' families. An anesthetist, surnamed Lee, admitted that she placed nine bottles of Atracurium in the baby ward's refrigerator that also stored the vaccines. She explained that she thought the Atracurim would come in handy when pregnant mothers needed operations. Maintaining that a warning sign was placed on the bottles, she regretted the mistake.
"Nurses should triple check the drugs before making injections," the Central News Agency quoted Lee as saying. But the county's health bureau chief, Lee Lung-teng, came up with a conspiracy theory, saying human error was "unlikely." He said Atracurium did not need refrigeration, and the drug was contained in 2.5 cc bottles. He said the sizes and labels of the two kinds of containers differed a lot, and it would have been impossible for the nurse to misidentify one for the other, and give seven injections without knowing. He said he did not rule out the possibility of the hospital "being set up." DOH head Twu Shiing-jer said the health authorities would provide the families of the victims with help should there be any litigation against the hospital.
http://www.nytimes.com/2002/02/16/international/16BRAZ.html
Merck Says Tens of Thousands May Need Another Hepatitis Shot
Merck & Company said on Friday that an unknown number of people in as many as 27 nations, including 60 000 youngsters in Brazil, might need new shots to prevent infection with the hepatitis A virus because vaccines they received might have been defective.
A unit of the French pharmaceutical group, Aventis, which sells the vaccine in Europe in a joint venture with Merck, disclosed in December that it was recalling batches of pre-filled syringes because they might not be potent enough to protect against the virus, which is spread by poor sanitation and can cause liver damage. At the time, however, neither Merck nor the Aventis Pasteur unit indicated how many people who had taken possibly faulty batches made between December 1999 and December 2001 would need new vaccines.
Although the batches may have been ineffective in protecting against the virus, Merck said the vaccines were not harmful. Gwen Fisher, a spokeswoman at Merck's headquarters in Whitehouse Station, N.J., said on Friday that the possibly ineffective shots of its hepatitis A vaccine given in the past 2 years include both the VAQTA K vaccine for children and the VAQTA vaccine for adults.
"Merck is offering to pay in most countries for either retesting people to see if they are effectively vaccinated or for revaccinations," said Ms. Fisher, who added that she did not know Merck's potential financial liability. Merck commented on the potential number of people affected by the faulty vaccine in response to inquiries by Reuters following reports by local newspapers in Brazil about the use of the possibly ineffective vaccine in Brazil.
Merck sells the VAQTA K vaccine against hepatitis A for young people in the United States, Latin America, Asia, and parts of Europe. The vaccine is put into syringes in Britain by Evans Vaccines, part of PowderJect Pharmaceuticals. In Brazil, a spokesman for Merck's unit there, Merck Sharp & Dohme, said about 60 000 children and adolescents in the country could need another dose of the preventive drug. Merck said the young Brazilians were among those who took about 117 000 possibly faulty vaccines in the past 2 years, mostly in private clinics in the industrialized states of Sao Paulo, Rio de Janeiro, and Minas Gerais. Each dose of the vaccine was given twice to the Brazilian youths and, although not harmful, it may not be potent enough to prevent the disease, the company said. "The reaction varies from person to person and we cannot guarantee that the doses in the problem lots will be effective," said Marcos Levy, director of corporate affairs for Merck in Brazil. Mr. Levy said oxygenated water had seeped past the seal in pre-filled syringes of the medicine packaged in Britain and also sent to the United States, France, Ireland and Germany. "We recalled that lot and other lots that, although still valid, used the same type of seal on the syringes," Mr. Levy said. He added that the Brazilian clinics that gave the vaccine are contacting patients so that they can return for testing and perhaps a new shot. Unlike hepatitis B or C, hepatitis A is rarely deadly and only severe in about 2 percent of cases.
Manitoulin residents get shots of wrong flu vaccine
http://www.thesudburystar.com/webapp/sitepages/content.asp?contentid=14491&c
atname=Local+News
By Star Staff
Wednesday, November 20, 2002 - 11:00
Local News - As Homer Simpson might say, "Doh!"
The Sudbury and District Health Unit announced Tuesday it has learned that some of last year's influenza vaccine was inadvertently included in this year's shipment from the Ontario Government Pharmacy. As a result, people on Manitoulin Island who have already received their flu shot may have to have a second shot to be fully immunized. Using a prior year's vaccine is safe, but may result in inadequate protection for the strain of flu expected to circulate in the current year, the health unit said in a news release. "The Sudbury and District Health Unit has reviewed its records and identified that two clinics on Manitoulin Island used last year's vaccine," the news release said.
"These individuals have already been contacted directly by the health unit and advised to obtain a second flu shot." The health unit also said it has contacted all health-care providers who received flu vaccines this year. Health-care providers were asked to check their vaccine stock and to review immunization records. The health unit requested that anyone identified as having received last year's vaccine be notified directly by the health-care provider.
"If people were immunized with last year's vaccine, they may be only partially protected from the influenza viruses expected to circulate this year," said Kelly Reilly, manager of clinic services with the Sudbury and District Health Unit. "That is why we recommend re-immunization for anyone who is informed that they received last year's vaccine," Reilly said. "Getting a second flu shot is safe and will ensure that people are appropriately protected against influenza this season." The health unit said it will closely monitor the response to its letter to health-care providers and offer assistance as needed. Hope you enjoyed reading The Sudbury Star online. Click here to order convenient home delivery.
Baby, eight weeks, given MMR vaccine Dec 13 2002
http://icwales.icnetwork.co.uk/0100news/0200wales
/page.cfm?objectid=12451367&method=full&siteid=50082
By Owen Fairclough, PA News
A doctor's surgery was today carrying out an inquiry into how an eight-week-old baby received the controversial MMR jab by mistake. Shannon Whitter was given the mumps, measles and rubella vaccination by a nurse at the Bellevue Medical Centre in Birmingham instead of diphtheria, whooping cough and tetanus. The tot has so far suffered no adverse effects from the wrong jab, which is normally GIVEN to youngsters at 12 months. Critics of MMR claim it can lead to autism and irritable bowel syndrome in young children.
Shannon's father, George Whitter, 43, said: "Fortunately, Shannon seems to be fine. "But we're keen to see the outcome of this inquiry because another child may not have been so lucky." Shannon's mother, Christine Fullen, took Shannon to the surgery for her inoculations on Tuesday. After a nurse carried out the vaccinations, they returned to their home in Acorn Grove, Ladywood, - and Miss Fullen found a message on her answering machine from the surgery reporting the blunder. Shannon was taken to the Diana, Princess of Wales Children's Hospital in Birmingham, where doctors told her parents that she was protected against any adverse effects because her mother's antibodies was still in her system.
Miss Fullen said: "We are really angry about this because it should not have happened in this day and age." Dr Andrew Carson, of the medical centre, said the nurse involved would not be administering any further vaccines. "We are looking further with the practice team into what exactly led to this error and procedures will be reviewed to prevent this from happening again," Dr Carson said. "We deeply regret this incident and the concern this has caused to the family." Dr Carson added that manufacturers of the MMR vaccine advise that it is safe to administer it earlier than 12 months, in the event of an outbreak of measles, for example.
Warning over 'useless' mumps vaccine
Dec 3 2002
http://icberkshire.icnetwork.co.uk/0100news/0400bracknell
/page.cfm?objectid=12422754&method=full&siteid=50102
By Colin George
DOCTORS are warning Bracknell parents to be on the look out for a grey-market mumps vaccine which could be useless against the contagious disease. The single jab imported from the Czech Republic is not used by the NHS, but may have been injected into children at private clinics in the area. And youngsters who received the faulty cure may now have to take the controversial combined measles, mumps and rubella vaccine, which some claim to be linked to autism. Medical experts also believe nearly 6,000 doses of the drug Pavivac - which is not licensed by the Government's Medicines Control Agency - are sitting in surgeries waiting to be used. If the vaccine is not kept at a precise temperature it can break down, leaving children unprotected from a range of symptoms including swollen glands, sore throats and fever.
In extreme cases the infection can go on to cause deafness, viral meningitis and sterility in adults. Medicine safety committee chairman Professor Alasdair Breckenridge said: "There are a number of major questions about the manufacture, testing and storage of the unlicensed vaccine Pavivac which are not answered by the information currently available. "Because of this lack of information we are advising its importation and use should be halted as a precautionary measure, and we have also urgently asked for further information and clarification." racknell GP George Kassianos said children who received the suspect vaccine should be given the controversial MMR jab to ensure they are protected. He said: "It is perfectly safe to repeat the dose. Children or adults who have had a single vaccine previously are either immune and unlikely to suffer side effects, or are not immune and need the vaccine."
Published 12/3/2002 http://www.lsj.com/news/local/021203_vaccine_1a-6a.html
Meningitis vaccine might not halt disease
MSU contacting students who got vaccinated
By Sharon Terlep
Lansing State Journal
EAST LANSING - MSU is sending letters to 2,300 students who received meningitis vaccines, after the manufacturer said the inoculations might not work.The nation's only producer of the adult version of the vaccine says it may not ward off a strain found in certain parts of Africa or in laboratories that study the disease.
Vaccine recall
Some meningitis vaccines made between January 2001 and October 2002 have been recalled. People should consider being revaccinated if they received a vaccine in that time and: Work in a laboratory or industry that exposes them to the meningococcal group A. Travel to parts of the world known at the "meningitis belt." This includes parts of Benin, Burundi, Burkina Faso, Cameroon, Chad, Ethiopia, Gambia, Ghana, Mali, Niger, Nigeria, Rwanda, Senegal, Sudan and Tanzania. People who may need a new vaccine should call the place where they received their first one. Revaccinations will be paid for by the manufacturer. On the Web www.menomune.com/page2.html
Michigan State University has Study Abroad programs in Ghana and Senegal - where people could be at risk. But most MSU students are not at risk for meningitis, which is an inflammation of the lining surrounding the spinal cord and brain. The school is sending the letters as a safeguard. "The reality is there aren't a large number of students who travel to Sub-Saharan Africa," said Kathi Braunlich, communications and planning coordinator at MSU's Olin Health Center. "But we want people to be well informed." The faulty vaccines date to January 2001. They're produced by Aventis Pasteur in Bridgewater, N.J. The firm will pay for revaccinations among people who are at risk, spokesman Len Lavenda said. He wouldn't say how much vaccine the company produces or how much might not work. He said, in some cases, tests by the company revealed the vaccine might not protect against Group A of the meningococcus bacteria - found in a strip of African countries known as the "meningitis belt." The illness affects nearly 3,000 Americans a year, though there's been only one death involving the group A strain in the last decade, according to Aventis. "This is not a safety issue and no one needs to be concerned about it," Lavenda said. The Ingham County Health Department is sending about 50 letters to people who may have received the faulty vaccine, Medical Director Dean Sienko said. Shiawassee Country put out a notice Monday offering free revaccinations to people in the affected groups.
At Central Michigan University in Mount Pleasant, 70 miles north of Lansing, a few students have come in for another vaccine, officials said. Meningitis is an issue on college campuses because students living in close quarters are more likely to become sick. It's spread through intimate or household exposure such as kissing, sharing eating utensils or by secretions from the nose and throat. The bacteria have infected six MSU students in the past five years. Three died.
Contact Sharon Terlep at 377-1066 or sterlep@lsj.com.
New military recruit died of meningitis shortly after being vaccinated for it.
http://story.news.yahoo.com/news?tmpl=story&u=/ibsys/20021224/lo_kgtv/1432569
Officials: Recruit Did Not Die Of Strep A
Tue Dec 24, 1:56 PM ET Add Local - KGTV TheSanDiegoChannel.com to My Yahoo!
A Marine recruit who died Dec. 15 had an overwhelming meningococcal bacteria infection that was different from the streptococcus A that infected 185 other recruits at the Marine Corps Recruit Depot, it was reported Tuesday. No other recruits at the MCRD have shown symptoms or have been diagnosed with a meningococcal infection, Capt. John Malone, medical services director at Naval Medical Center San Diego, told the San Diego Union-Tribune. It was purely chance that the two separate bacterial infections, meningococcal and step A, hit the recruit population at the same time, Malone said.
Other members of Pvt. Miguel Zavala's platoon received a special oral antibiotic the day he died that should safeguard them against the bacteria, Malone said.
Doctors did not give the antibiotic, called levofloxacin, to all 4,500 recruits and depot staff because no one else showed symptoms of the rapidly moving infection that killed Zavala, Malone said. Recruits in other platoons and the public are not at risk because the bacteria is only spread to others in the same living area, Malone said. "You have to be in the same household," Malone told the Union-Tribune. "You don't get it by just walking across the parade ground." All recruits entering MCRD are vaccinated against the meningococcal bacteria, but the vaccine is not always effective, Malone said. One recruit remains in critical condition from the step A-related pneumonia outbreak that struck the depot. More than 126 people were hospitalized with pneumonia, though not all were related to strep A.
On a side note, if I had access to the "phials" or "vials", I'd throw them overboard too.
http://www.guardian.co.uk/uk_news/story/0,3604,879153,00.html
Rebecca Allison
Tuesday January 21, 2003
The Guardian
The Ministry of Defence has launched an internal investigation into how dozens of phials of anthrax vaccine were found washed up on a south coast beach yesterday. The packages of ampoules, which were discovered at West Bay, Dorset, posed no risk to health or the environment, according to the MoD.
"There are ampoules of anthrax in containers on the beach but they are not posing a risk to public health. We are working together with local police to make sure the vaccines are disposed of safely," a spokesman said. Anthrax, a biological agent, is one of the main concerns of the UN biological weapons teams searching Iraq. It is believed that the investigation into the discovery of the ampoules will include looking at whether they came from a warship involved in the recent Navy taskforce deployment to the Gulf headed by HMS Ark Royal. Dorset police said the alarm was raised at 11am by the Bridport harbourmaster after a sighting of unidentified packages on the beaches at West Bay. "The phials were confirmed as anthrax vaccine and we were advised they were sterile and contained no anthrax. The services worked to collect the packages.
"As the work was under way other packages, also found to be a medical substance, were washed up and disposed of in chemical bins," a spokesman said. The MoD confirmed that the packages contained two types of phials. One type contained the anthrax vaccine and the other contained phials of a drug called dimercaprol which acts as an antidote to heavy metal poisoning. The packages consisted of individual sealed ampoules which were packaged in sealed plastic boxes and wrapped in polystyrene inside cardboard boxes, the spokesman confirmed.
He said the batch numbers of the anthrax vaccines had been checked and were found to have been manufactured at the Centre for Applied Microbiological Research at Porton Down. "We haven't been able to search the source of the vaccine, but we can confirm it was issued to the armed forces. There is an internal investigation under way to find out how the ampoules came to be in the water," he added.
Friday, February 7, 2003
1,900 got expired measles vaccine (in 1993)
Friday, February 7, 2003 at 09:30 JST
TOKYO — As many as 1,900 children may have been given shots of a measles-mumps-rubella (MMR) vaccine that had already passed its expiry date during the seven months up to the time such vaccinations were banned April 1993, Kyodo News learned Thursday. The Health and Welfare Ministry, the predecessor of the current Ministry of Health, Labor and Welfare, apparently did not disclose the use of the expired vaccine or report it to a sub-panel of the ministry's Council on Public Health that had been discussing rampant cases of the MMR vaccine's side effects.
A group supporting victims of the vaccine's side effects alleges that the government deliberately covered up the expired vaccine's use. An expired vaccine "will not lose its effectiveness immediately or raise the risk of side effects," a ministry official said, but added, "The use of expired vaccines is naturally a problem. We would like to look into the case to find out why that happened. "The vaccine was introduced in 1989 to protect children from the three diseases in a single shot. Production of the stock used for the MMR vaccine was banned in 1991 after it caused side effects in a large number of children. The expiry date for the vaccine was September 1992.
According to vaccine reports that prefectures submitted to the health ministry at the time and other materials, 1,829 people were given the vaccine in eight prefectures between October 1992 and April 1993. Kumamoto Prefecture saw the largest number of people who had the expired vaccine at 801, followed by Hokkaido at 318. Data gathered by members of the predecessor body of the National Institute of Infectious Diseases show that in Tokyo and Kanagawa, two prefectures with no existing records on MMR vaccine use, a total of 104 people were given the vaccine. In all, 1,933 received the vaccine over the period from October 1992 through April the following year.
The health ministry said, however, that the figure may include people who had the vaccine before September, the month it expired, noting that some reports may have come in late. The ministry recently found an in-house document showing it had received reports on five boys aged between 1 and 4 who developed aseptic meningitis after receiving the expired MMR vaccine, but it had kept the fact concealed. A court ruling is expected in March on a suit filed by people who remain disabled due to the vaccine's side effects and the families who lost their children because of the side effects.
Being open and honest defused a bad blunder
Pulse; Tonbridge; Jan 13, 2003;
Full Text:
Copyright CMP Information Ltd. Jan 13, 2003
MMR vaccine; DPT vaccine
Dr Andrew Carson examines the aftermath of a vaccine given in error
The child health clinic had been much like any other, apart from the fact that the regular nurse was away on an immunisation update course and her place had been taken by another practice nurse. The clinic finished just before evening surgery. Suddenly, the relative tranquillity of this interval was shattered by the appearance of the nurse in a state of some distress, come to inform me that she had inadvertently given an eight-week-old the MMR vaccine in place of the DPT and HiB. Discussion with the nurse and health visitor ensued, and the health visitor agreed to contact the family as soon as possible. I did not believe there was any increased risk to the child from the MMR vaccine being given early, but checked this with public health officials and the manufacturers. Both sources confirmed my initial assessment. Towards the end of evening surgery I heard from the parents, who expressed concern and anger. I apologised on behalf of the practice and reassured them that their child was not at any increased risk from the early administration of the vaccine. These points would need to be reiterated frequently over the coming days, at each contact with the parents.
Opportunity for discussion
I invited the parents to discuss things with me as soon as surgery had finished. The discussion was lengthy and covered their distress and concern over the fact their child had received a controversial vaccine without their consent. I felt my function at that time was to listen and be supportive without being defensive. The family have subsequently said that, although the incident should not have happened, they felt very supported through their anxieties by the actions of the practice team.
I concluded my interview with the parents by informing them about our complaints procedure and by giving them details of how I could be contacted personally at all times over the coming days. I contacted them later that night and early the following morning to check that the baby was well. The following morning the sequence of events was reported to our primary care manager who immediately started a Serious Untoward Incident investigation. This involved interviewing all the parties involved, including the parents, to try to establish the cause of the incident and see what additional safety measures could be put in place before the next clinic. The PCT was also informed early in the day.
By late morning on day two we were informed that a relative of the baby had approached the press, and we were asked for a statement by a daily newspaper. After obtaining consent from the baby's parents, a press release was prepared in conjunction with the PCT. The media agency employed by the PCT was invaluable at this stage, fielding much press attention. Misreporting and misrepresentation made the front page in the local paper that afternoon. For example, it was reported that the baby had been rushed to hospital, which hadn't been the case at all. This was followed by a request for an interview by the local television news. The media agency was again extremely helpful in preparing me for the questions.
Vaccinations as usual
We felt it was important to stress the support we were giving to the baby's family, the nurse involved, and the rest of the practice team. Furthermore, it was crucial to reinforce the importance of parents continuing to allow their children to be vaccinated in the usual way. The incident was, after all, a rare and isolated one that had not put the baby involved at any increased risk.
Subsequent activity revolved around accurate documentation and reporting of events surrounding the incident, as well as implementing new safety procedures and issuing a statement to our patients. The MPS had been involved from an early stage and appeared happy with the way we had handled the situation. We also sought advice on catching up with the DPT vaccine that had not been administered. Finally, the nurse involved had to go through a disciplinary hearing.
It was a frantic few days that involved a great deal of upset for everyone involved. But it was also a valuable learning experience and much good has come out of it. For example, we now plan to colour- code our vaccines for easy identification.
The nurse, along with the rest of the team, behaved with great honesty and integrity once the mistake had been discovered. This cannot be stressed too strongly. One hopes that aggressive press attention will never discourage individuals from admitting their mistakes. I am convinced that our being open and honest helped defuse a difficult situation.
Andrew Carson is a GP in Birmingham
Practice candid over MMR error
Pulse; Tonbridge; Jan 6, 2003;
Full Text:
Copyright CMP Information Ltd. Jan 6, 2003
MMR vaccine; DTP vaccine
Dr Andrew Carson found openness was the best policy when his practice nurse accidentally gave an eight-week-old the MMR vaccine. Dr Carson, a GP in Birmingham, commended the nurse for behaving 'extremely responsibly' by informing them immediately when she realised she had given MMR instead of DTP vaccine. Packaging confusion could be to blame. Dr Carson said: 'Once the vial is taken out of the pack the name of the vaccine is actually obscured by the lot number and expiry date. But that is not to excuse what happened.'
Copyright: CMP Information Ltd.
http://news.scotsman.com/latest.cfm?id=5731551
12:31am (UK)
Private Clinics Botched Children's Vaccines - Report
By Pat Hurst, PA News.
Hundreds of children may have been put at risk after two private clinics botched vaccinations for measles, mumps and rubella, it emerged today. Worried families have been told that single shot immunisations given to toddlers at two private clinics, one in Sheffield and one in Hertfordshire have not been done properly. It means children could pick up infections and may not after all be inoculated against the childhood diseases of measles, mumps and rubella. And the vaccines themselves may have become contaminated, leading to an increased risk of children suffering bacterial infections, experts warned. The parents paid the private clinics for their children to receive single injections of the vaccines. Some parents claim the three-in-one MMR injection delivered by the NHS can cause autism and Crohn's Disease. Instead of getting the NHS recommended MMR vaccines they paid around £70 for the single vaccines, according to the Mail on Sunday.
The clinics are run by Lifeline Care Ltd.
At the clinic held at the Hillsborough Sports Arena in Sheffield, 718 children were given the faulty vaccines, while 295 were given the single dose vaccines at the Elstree Aero-Medical Centre in Hertfordshire. The faulty vaccines were given at both clinics between June and December of last year. Most of the children are toddlers. The problem arose because the clinics changed the normal procedure for making up the vaccines, according to the local NHS trust in Hertfordshire. They began pre-preparing batches of vaccines so more children could be treated which investigators think led to the vaccines not working properly. It means potentially hundreds of children were not then protected from the diseases. The error only came to light after two doctors, who worked at the clinic in Hertfordshire, left and wrote a confidential letter to the local Hertsmere Primary Care Trust, which then investigated.
Dr Joel Bonnet, director of public health at the trust, said, "As a result of the changes in the way the vaccines were made up there is a possibility that the efficacy of the vaccine has been effected, so that children are not as protected as normally they would be. "There is a potential risk, which is why we are recommending parents get the children re-vaccinated with the MMR." Dr Bonnet said he was not aware of any of the children who were not properly vaccinated, subsequently falling ill with measles, mumps or rubella.
The clinics concerned defended their actions.
Dr David Pugh, medical director of Lifeline Care, told the Mail on Sunday they followed "common practice" when making up the vaccines. "During last year we had particularly busy clinics and decided to reconstitute the vaccine in advance," he said. "The vaccines were used within the six-hour time scale recommended. The view of the Department of Public Health was that the potency of the vaccine could not be guaranteed in those circumstances." The two clinics concerned will be investigated by the National Care Standards Commission which is the watchdog for all private medical clinics. The General Medical Council will also investigate what went wrong. The clinics are still in operation but have now reverted to making up the vaccines as recommended by the manufacturer. The Hertsmere Trust has written to all the families of the 1,013 children effected to tell them their children may not be properly protected.
It recommends that all children get the MMR vaccine. Any parents who think their child might be effected can ring NHS Direct on 0845 4647. Also more information on the MMR vaccine is available at the following websites:
www.immunisation.org.uk
www.mmrthefacts.nhs.uk
www.phls.org.uk
I guess monkeys, chicken embryos, aborted fetal tissue, and all that is Ok, but my gawd........not a dog. We all know its political, isn't it. BUT again, NO vaccine is safe, including this one. Its just their hypocrisy.....
Sheri
Mumps vaccine suspended in UK
http://www.praguepost.com/P03/2003/Art/0122/news6.php
Safety group concerned that materials in drug present infection risk
By Mindy Kay Bricker
Staff Writer, The Prague Post
(January 22, 2003)
What is good enough for the Czech Republic is not necessarily good enough for the United Kingdom. That was the message from a group of British medical officials who announced Jan. 16 that the United Kingdom would suspend the importation of Pavivac, a Czech-made mumps vaccine for children.
"There are a number of outstanding questions about the manufacture and testing of the unlicensed vaccine Pavivac that are not answered by the information currently available," said Alasdair Breckenridge, chairman of the Committee on Safety of Medicines (CSM), an independent scientific committee that advises the government on medicines. The committee said it was rejecting the vaccine because kidney cells from dogs are used to make it. The CSM said that using materials of animal origin in humans might present a risk from unknown infections.
"There are no other vaccines in the Department of Health's vaccination program which use this method of manufacture," reads a CSM press release. "As a result, there are a number of additional questions not all of which have been satisfactorily answered." Miroslav Reinhardt, export director for Pavivac-maker Sevapharma, said the vaccine was safe and that his company has been cooperative with UK officials. He said the kidney cells were taken from dogs bred at farms that follow laws regulating such practices.
"We cooperated with the Medicines Control Agency (MCA) intensely -- we provided them with our documentation," he said, "and we hope they will reconsider their decision." The vaccine was not licensed for distribution in the United Kingdom. If a medicine meets the special needs of individual patients, doctors in the United Kingdom are allowed to obtain unlicensed drugs. First, however, the drug must be approved by the MCA, the executive agency of the Department of Health that ensures that all drugs meet the appropriate standards of safety, quality and efficacy.
From June to November, more than 5,000 doses of Pavivac were sent to the United Kingdom. Physicians directly requested the vaccine from Sevapharma. Pavivac first came under fire in the United Kingdom in November, when the CSM suspended importation of the vaccine. At the time, some British doctors criticized the action, calling the suspension "appalling scaremongering." When the vaccine was suspended, Czech health officials said they tried to comply with the British government's demands for more information about Pavivac.
"Nothing [about the demands] was very specific or indicated that the product was substandard," said Milan Smid, a doctor for the Czech Regulatory Authority, the government agency that regulates drugs. The agency reviewed the product, Smid said, and did not find any reason to discontinue or halt production in this country. "We didn't have any indicator to take action against the product here," he said. Reinhardt said that no one who has taken the vaccine, which has been used for 14 years, has experienced any serious adverse reactions. "More than 1.4 million children have been vaccinated with no problem," he said of the single mumps jab used in the Czech Republic.
Smid agreed.
"Through the years of use, we had a normal spectrum of the side effects that is comparable to other vaccines," he said. "There is no reason to take actions against this vaccine." United Kingdom health officials said they were unsure how many of those doses imported into their country were administered to children. The CSM has asked clinics to provide a record of children who received the jab so that any adverse reactions can be quickly and thoroughly investigated. Breckenridge said that Sevapharma provided adequate information that proved Pavivac is effective in protecting children against mumps, as long as the child receives a second mumps vaccine between six to 10 months after the initial shot. Currently, Pavivac is sold only in the Czech Republic and is not licensed in any European Union country. The company also manufactures a single measles vaccine, Movivac. British health officials are investigating its safety, even though the measles vaccine has not been imported into the United Kingdom.
Mindy Kay Bricker's e-mail address is mbricker@praguepost.com
Family in new jabs scare
Feb 12 2003
By Ed Reed, The Huddersfield Daily Examiner
LITTLE Oliver Wilson may have to have more vaccinations after new health fears. The Waterloo toddler faces more injections after concerns that separate measles, mumps and rubella jabs given to babies at a private clinic in Sheffield may not have worked. Lifeline Care, which offered single injections at clinics held in Hillsborough Arena last year, failed to meet standards set down by a national watchdog. Now, Alison Wilson, aged 38, and husband John, 37, of Sunny Mead, do not know if 20-month- old Oliver is protected. Oliver went for a measles jab at the clinic last July and a rubella vaccination in October. His parents, who queued for three hours on their first visit to the clinic, wanted Oliver to have separate jabs after hearing how the triple MMR vaccination has been linked the development of autism and the bowel disorder Crohn's disease in toddlers. Single jabs are not available on the NHS. Mrs Wilson found out about the Lifeline Care clinics on the internet. Each injection cost £75. But this week they heard the vaccine given to their son may not have been properly prepared. Oliver, who is recovering from chickenpox, will now go for a blood test to determine whether the vaccines have worked. Sadly, the test is not foolproof and his concerned parents are unsure as to whether they should repeat the jabs. "You pay and put your trust in these people and then this happens," said a worried Mr Wilson. He and his wife were eager to get Oliver vaccinated, but not with a triple jab. "Our best course of action was to go with the single vaccinations," said Mr Wilson. "When I was a child we got single vaccinations. Parents should be able to say whether they want their children to have triple or single jabs." Lifeline Care goes against normal procedure and uses pre-prepared vaccines at its clinics. Health watchdog the National Care Standards Commission has investigated the Lifeline Care sessions and ordered the company to improve its practices and staff training.
Letters are being sent to the parents of 718 children who received vaccines at the Sheffield clinic between June and December, telling them of the problem. Mr Wilson questioned whether all children were affected, or just those who received a certain batch. He criticised Lifeline Care who, he said, had offered no information to worried parents. "They haven't come out with a statement to say whether it was particular batch. All we know is it's the vaccine distributed between June and December." He never remembered being asked for his address by Lifeline Care. Parents with any concerns about their child's vaccination should contact their GP or NHS Direct on 08457 4647.
Parents told to get babies a second MMR jab
By Ian Lloyd
http://www.thisishertfordshire.co.uk/news/barnet/display.var.696255.
index.parents_told_to_get_babies_a_second_mmr_jab.html
More than 40 children from Barnet are at greater risk of catching measles, mumps or rubella after it emerged their doctor is under investigation for giving them faulty vaccinations. Hertsmere Primary Care Trust (PCT) is urging parents whose children were given the MMR jab by GP Dr David Pugh at the Elstree Aeromedical Centre, Elstree Aerodrome, to get them treated again.
The PCT believes 295 children, including 44 from Barnet, 88 from Hertfordshire and 55 from Middlesex, are at risk after Dr Pugh gave them vaccinations prepared several hours before they were due to be administered against the manufacturer's guidelines. The children were treated between June and December last year. "Some children who have been vaccinated may not be adequately protected against one or more of the diseases," said the PCT's director of public health, Dr Joel Bonnet.
"Due to the way the vaccines were prepared, they may have become contaminated." Such contamination would increase the risk of bacterial infections or side effects to the vaccine, he said. While he could not deny he had prepared vaccinations earlier than he was supposed to, in a letter sent to parents Dr Pugh said he had done nothing wrong. "No parent has ever filed a complaint the only reactions notified are normal reactions to vaccinations and none serious," he wrote.
Investigations into standards at the clinic were started after two former members of staff wrote to Hertsmere PCT claiming vaccines were being prepared incorrectly. The clinic is now being investigated by the National Care Standards Commission (NCSC), which monitors standards at private health practices. The NCSC is also looking into Dr Pugh's claims to have administered 9,000 doses of the unlicensed drug Secretin to 1,500 autistic children.
Dr Pugh has been administering the drug since 1998, although there is no information available about its long-term effects.
http://news.sify.com/cgi-bin/sifynews/news/content/news
_fullstory_v2.jsp?article_oid=12631678&category_oid=-20611&page_no=1
Kids given Insulin instead of Hepatitis B vaccine
Thiruvananthapuram, Feb 20
In a glaring case of medical negligence, about 400 children who were mistakenly administered insulin instead of hepatatis vaccine were rushed to the Medical College Hospital in Thiruvananthapuram from suburban Kalliyur on Wednesday evening. Hospital sources said that the children were kept under observation, though the condition of none of them was stated to be critical. "We continue to receive children from the locality as all the children who took the shot today are being asked to get admitted in the hospital," the sources said. Official sources said the vaccination drive was carried out at the Kalliyur Primary Health Centre, near Peringammala In a glaring case of medical negligence, about 400 children who were mistakenly administered insulin instead of hepatatis vaccine were rushed to the Medical College Hospital in Thiruvananthapuram from suburban Kalliyur on Wednesday evening.
Hospital sources said that the children were kept under observation, though the condition of none of them was stated to be critical. "We continue to receive children from the locality as all the children who took the shot today are being asked to get admitted in the hospital," the sources said. Official sources said the vaccination drive was carried out at the Kalliyur Primary Health Centre, near Peringammala.
http://www.thisislocallondon.co.uk/news/
display.var.702494.Top+Stories.clinic_at_
centre_of_child_jab_scare_is_forced_to_close.html
Clinic at centre of child jab scare is forced to close By Ian Lloyd
The clinic at the centre of an investigation into whether it gave hundreds of children ineffective single inoculations against measles, mumps and rubella has been shut down. Lifeline Care Limited, which runs the Elstree Aeromedical Centre at Elstree Aerodrome, was ordered by the National Care Standards Commission (NCSC) to cease practising from 5pm on Friday last week. The clinic, which offered single jabs to combat measles, mumps and rubella, is not registered with the NCSC and was therefore operating illegally. Hertsmere Primary Care Trust (PCT) and the NCSC are currently investigating single-dose jabs given to children at the centre between June and December last year.
Some 295 children including 40 from Barnet may be affected. Meanwhile, there have been unconfirmed reports that a 19-month-old boy, who had the single jab vaccinations at the centre in September, has caught measles. The boy's mother had been told by doctors at Great Ormond Street Hospital that there was a 'strong possibility' he had contracted the disease. A spokeswoman for the NCSC, which monitors private clinics, said: "We have been told by several parents that their children seemed to have contracted measles. But you do sometimes get a mild form of the disease after the inoculation."
Sharon Gold, of Anthony Road, Borehamwood, was horrified when results of a blood test showed her son, who was treated at the clinic, was not immune to any of the diseases. "I am absolutely in shock," she said. The PCT is urging parents who believe their children may have contracted measles, mumps or rubella after being vaccinated at the clinic to write to Dr Joel Bonnet, Director of Public Health, Hertsmere Primary Care Trust, The Elms Clinic, High Street, Potters Bar, Herts EN6 5DA with full details.
http://www.bangkokpost.com/News/03Mar2003_news19.html
Hospitals given vaccines alert after infant's death
Aphaluck Bhatiasevi
State hospitals have been told to closely monitor any adverse effects from vaccine immunisations, following the death of a four-month-old infant this year. Though investigations did not confirm that the boy's death was due to vaccines, health authorities have not ruled out the possibility. Since 1997, there have been five confirmed deaths of children younger than five due to vaccines, according to the Diseases Control Department.
The investigating team, which published their findings in a recent Weekly Epidemiological Surveillance Report, said that although no other child who received the same vaccines suffered any serious side-effects, there was a possibility that the boy could have died from the vaccines. The boy was healthy when taken for a regular dose of DTP and OPV vaccines for diphtheria, tetanus, pertussis and polio at Tha Rua district hospital in Nakhon Si Thammarat on January 15 at 11.30 am. His mother found him dead at 8am the next day.
He had big red patches on the back, under his skin. Investigating authorities did not rule a vaccine-related death because his mother suffered from syphilis during his birth, for which both were treated for 10 days. From 69 children under five treated for side-effects from immunisation at Tha Rua district hospital between October last year and January 21 this year, 18 had pneumonia, 17 suffered from fever while 14 had diarrhoea. Public health authorities have been told to carefully monitor storage procedures and the use of vaccines to ensure safety for children below five years of age.
IVAX Pharmaceuticals Has Recalled ONXOL injection
(SafetyAlerts) - The Food and Drug Administration (FDA) released the following information.
PRODUCT
ONXOL injection (paclitaxel), 300 mg/50 mL (6mg/mL), 50 mL Multi Dose
Vial, Rx only. Recall # D-161-3.
CODE
M026861 Exp Date 02/04.
RECALLING FIRM/MANUFACTURER
IVAX Pharmaceuticals, Miami, FL, by letter on January 20, 2003. Firm initiated recall ongoing.
REASON
Lack of assurance of sterility: Environment in Class 100 Filling room exceeded the non-viable particulate limit specification.
VOLUME OF PRODUCT IN COMMERCE
1,039 vials.
DISTRIBUTION
Nationwide.
Eli Lilly Has Recalled GEMZAR for Injection
(SafetyAlerts) - The Food and Drug Administration (FDA) released the following information.
PRODUCT
GEMZAR for Injection, (Gemcitabine HCl), 200 mg, For I.V. use only, 10 mL
Sterile Single Use Vial, Lilly, Rx Only, Vial No. 7501. Recall # D-157- 3.
CODE
Lot 6MH15.
RECALLING FIRM/MANUFACTURER
Eli Lilly & Company, Indianapolis, IN, by letter on or about December 20, 2002.
Firm initiated recall ongoing.
REASON
Container Defect: glass in vials.
VOLUME OF PRODUCT IN COMMERCE
31,200 vials.
DISTRIBUTION
Agentina, Brazil, Columbia, Mexico, Taiwan and Venezuela.
[http://www.safetyalerts.com/_includes/general.htm]
Of course, the fact that these people are now being exposed to a double dose of adjuvants, heavy metals and toxic contaminants doesn't rate a word of concern, right?
Immunizations underway after vaccination mishap More than 170 babies and children have so far been re-immunised after a vaccination mishap in Ingham in north-east Queensland. About 400 hundred people have been recalled for immunisations received during the past 10 months, because vaccines were wrongly stored at the Ingham Hospital.
Anna Morgan from the Tropical Public Health Unit says authorities have contacted the parents of all but two of the 220 children involved and says the re-vaccination program should last another fortnight. About 30 adults are yet to be contacted to have their tetanus vaccinations again.
http://www.thisishertfordshire.co.uk/news/barnet/display.
var.703249.index.parents_looking_at_legal_action.html
Parents looking at legal action
By Ian Lloyd
Parents of children treated at the controversial Elstree Aeromedical Centre which offers single jab alternatives to the combined MMR vaccine are contemplating legal action against its director Dr David Pugh. Denise Goldsmith said she had been speaking to solicitors following confirmation that her 19-month-old son Noah contracted measles despite being given a £65 single jab at the clinic in September. Noah is one of 295 children who may have been given a faulty jab at the clinic in Hogg Lane, Elstree, between June and December last year.
The National Care Standards Commission, which closed the clinic down on February 20 for not having a licence to practice, is currently investigating the claim. "I am in the process of speaking to different solicitors and I have had about 100 calls from mothers who have had their children vaccinated at the clinic who all want to take it further," said Mrs Goldsmith, of Mill Hill.
"What happened to Noah was absolutely horrific I wouldn't wish it on anybody. The doctors didn't know if it was measles or not because it is so rare as children are vaccinated. "When he went into hospital with big purple spots they were treating him for meningitis for 48 hours." She added: "We sent Noah to the clinic because we didn't want to do the MMR vaccine. "We didn't want to play Russian roulette with his health and risk having an autistic child or a child with bowel disorder."
Dr Pugh, director of Lifeline Care Ltd, which runs the clinic, said he was unable to comment to the Times Group on the matter as his insurance company stated it would invalidate his medical insurance. A statement to parents from the clinic reads: "We have been in private medical practice for over 17 years and offering single vaccines for over five years. "Dr Pugh has been a GP for over 32 years and there has not been a single complaint filed against him."
18:27 Tuesday 4th March 2003
http://mdn.mainichi.co.jp/news/20030312p2a00m0dm004000c.html
Outdated vaccine injected into 1,000s of kids
Over 2,000 children were immunized a decade ago with unreliable vaccines known for causing side effects and well past their use-by date, the Ministry of Health, Labor and Welfare said Wednesday. Physicians across Japan continued to inject children with vaccines for mumps, measles and rubella even after the ministry had decided they no longer should be used. But ministry officials said there was no danger in them doing so. "They're still effective (vaccines) even if they are about half a year past their use-by date," a ministry spokesman said. Typically, the ministry's plan of action is more like one of inaction.
"Our probe into the situation has left us with the impression that individual physicians didn't pay sufficient care with what they were doing. We will set up a panel of experts and look into the correct and fundamental approach to take regarding immunization," the ministry spokesman said.
Ministry officials said the MMR vaccinations were given to children aged from 1 to 6 during the four years from April 1989. As the vaccinations were producing too many side effects, their maker announced in September 1991 that it would cease producing them. The medicines were said to be effective for one year. Ministry officials looked into how much of the outdated vaccines were used after October 1992, the time the ministry's supplies of the drugs reached the prescribed end of their effectiveness.
Going through documents of the only 10 prefectures still with paperwork from the time, the ministry learned 2,070 children had been injected with the out-of-date vaccines. (Mainichi Shimbun, March 12, 2003
Alzheimer's Vaccine Had Mixed Results
By RANDOLPH E. SCHMID
.c The Associated Press
WASHINGTON (AP) - The experimental vaccine withdrawn from testing after four Alzheimer's patients developed brain swelling seems to have reduced the accumulation of brain plaques associated with the disease but may have caused the dangerous inflammation, researchers report. The vaccine did not show success against another sign of the memory-destroying disease, tangled nerves and microfibers, according to the first autopsy results of one of the affected patients in the clinical trial. The 72-year-old woman died almost two years after first receiving the vaccine in July 2000.
The 360-patient trial was halted in January 2002 and the drug, AN-1792, made by Elan Corp. of Ireland, was withdrawn after the inflammation known as meningoencephalitis was found in four subjects. Doctors later discovered 11 more people with the symptoms. Doctors at the University of Southampton in England report that the autopsy results show that the swelling ``is likely to be a consequence of the immunotherapy.'' The vaccine had shown good results in mice, eliminating with few side effects the brain-damaging plaques called beta amyloid.
The accumulation of plaques associated with Alzheimer's seemed to have been cleared from large areas of the human patient's brain, they said. But they found no reduction in the tangled nerves and microfibers also present in the disease. ``That really couldn't have been predicted from the mouse studies because the mice develop only plaques and not tangles as they age,'' said James A.R. Nicoll, lead researcher on the report, appearing in Monday's online edition of the journal Nature. ``In Alzheimer's disease in general, we really don't know how much it is the plaques or the tangles that contribute to the brain dysfunction and dementia - or even if it is neither directly but that the dementia is due to loss of neurons or synapses from the brain,'' he said. The findings may give researchers a start to answering some of those questions, Nicoll said. ``This is the first time we have been able to separately affect plaques and tangles,'' he said.
Alzheimer's affects about 4 million people in the United States, and the number is expected to climb to 14 million by 2050 as the population ages. The disease was first described in 1906 by German doctor Alois Alzheimer and researchers have long sought a vaccine to combat it. There were high hopes for the vaccine when Elan d began testing, based on the results with mice.
In initial tests, humans had no adverse effects. But because the vaccine works by inducing the immune system to attack the protein that makes up those plaques, some scientists had warned that brain inflammation was a potential serious side effect. After halting the trial, Elan ended development of the drug, though the company said it still hopes to produce a vaccine for Alzheimer's using other approaches.
Bill Thies of the Alzheimer's Foundation said the paper is important because it indicates that the vaccine worked in helping remove plaques. That answers a vital question, whether a human given vaccine will generate enough antibodies to change the plaque concentration. ``Clearly the answer is yes,'' said Thies, foundation vice president for scientific and medical affairs. The autopsy results ``suggest an astonishingly powerful effect of the vaccination'' on removing plaques, added to a commentary by researchers at Massachusetts General Hospital who were not participants in Nicoll's report. But the data do not prove the effectiveness of the vaccine because it remains unknown if the symptoms improve after the drug's use, said the commentary by S.M. Greenberg, B.J. Bacskai and B.T. Hyman.
The woman autopsied had a five-year history of progressively worsening confusion and disorientation. She received her first vaccine in July 2000 and this was repeated after four, 12 and 24 weeks. A fifth injection was given at 36 weeks, using a reformulated vaccine. Six weeks later she suddenly had dizzy spells, drowsiness, unstable walk and fever, deteriorating to the point where tests could not be performed on her. She lived another year but showed no improvement in that time. Nicoll said there is no way to determine if the reformulated vaccine caused her sudden deterioration until other patients in the study have died and their brains are examined.
Nature Medicine: http://www.nature.com/nm
Unusual number of Nisswa School students out with chicken pox
NISSWA -- A higher than usual number of Nisswa Elementary School students may have chicken pox. Erin Suemnick, principal at the school, said 29 families have reported that their child has visible signs of chicken pox. Suemnick said in a letter to parents dated Tuesday: "Many of the Nisswa families are already aware of the unusual number of students that are at home already with chicken pox." Also in the letter, Suemnick wrote that the Brainerd Medical Center is interested in the inordinate number of chicken pox cases at the school. The medical center reported many of the children have already had the chicken pox vaccine. A questionnaire was developed to ask parents whether their children have been affected with chicken pox; if their child has had the immunization; or if the child has already been infected with chicken pox. Suemnick said she encourages families to call their doctor if they have any questions regarding their child's condition.
Date: Wed, 14 May 2003 00:15:04 EDT
From: GeoLith@aol.com
Subject: EuroStudy- No Link Between Unexplained Death and Vaccinations...
news about vaccinations, although it would be more reassuring if they were able to state the cause of death...
LONDON (Reuters Health) Apr 28 - The European Medicines Evaluation Agency said on Monday its scientific committee had found no evidence that combination pediatric vaccines were to blame for a number of sudden unexplained deaths. The Committee for Proprietary Medicinal Products reviewed the safety of Hexavac (Aventis-Pasteur MSD) and Infanrix Hexa (GlaxoSmithKline) after five reports of unexplained deaths in children occurring within 24 hours of vaccination. These vaccines offer millions of children protection against six serious life-threatening infectious diseases--diphtheria, tetanus, poliomyelitis, whooping cough, hepatitis B and Haemophilus influenzae b. "The causes of death remain unexplained and on the basis of available data, it is not possible to establish a cause and effect association with the hexavalent vaccines," the committee said in a statement.
"In several cases, sudden infant death syndrome, viral infection, metabolic disorders, allergic reactions or airway obstruction were plausible, however these could not be definitely proven to be the cause of death." Although a family history of epilepsy or convulsions at an early age was reported in three cases the committee decided that the clinical description of these cases did not provide sufficient evidence to identify this as a possible risk factor. It concluded that the benefits of vaccination far outweighed the possible risks of existing vaccines and that vaccination should be continued according to national vaccination schedules.
George Lithco
For info email us at SkipperVigil@yahoo.com
Shaking Kills: Instead Parents Please Educate and Remember If today is an average day in the United States, 4 children under the age of 5 will die or suffer permanent disabilities when they are shaken by a caregiver. Parents can prevent shaking injuries.
The "SKIPPER" Initiative
was formed by concerned parents to increase SBS awareness in the Hudson Valley and educate parents and caregivers about prevention. We are working with government agencies, local hospitals, day care providers, and community organizations to help parents and caregivers prevent shaking injuries.
http://money.iwon.com/jsp/nw/nwdt_rt.jsp?section=news&news_id=reu-n14382087&feed=reu&date=20030514&cat=INDUSTRY
Merck sued by Fox journalist over faulty vaccine
Wednesday May 14, 6:37 PM EDT
NEW YORK, May 14 (Reuters) - A Fox News journalist who contracted hepatitis A despite being inoculated before an assignment in Afghanistan sued Merck & Co. (MRK), the maker of the vaccine, on Wednesday. Merck recalled batches of ineffective hepatitis A pre-filled syringes in December 2001.
The journalist, Claude Novak, could be one of thousands of people who develop hepatitis A, which causes liver damage, after taking the faulty Merck vaccine. Unlike hepatitis B and C, the disease is rarely deadly. The suit, filed in New York State Supreme Court, claims Merck "negligently manufactured, distributed and sold the recalled vaccine which provided absolutely no protection against the disease," according to James, Hoyer, Newcomer and Smiljanich, the firm representing Novak.
A spokesman for Merck said the company has not seen the lawsuit and does not comment on ongoing litigation. He could not say whether this was the first time Merck has been sued in connection with the faulty vaccine. Last February the company said an unknown number of people in as many as 27 nations may need new shots to protect them against the disease because the
ones they received were faulty. A unit of French pharmaceuticals group Aventis SA, which sells the vaccine in Europe under a joint venture with Merck, said in December 2001 it was recalling batches of pre-filled syringes.
Merck sells a hepatitis A vaccine for adults, called VAQTA and one for children, called VAQTA K.
The recall covered vaccines administered after May 29, 2001.
http://www.reutershealth.com/archive/2003/05/01/eline/links/20030501elin019.
html
CDC reports 103 pregnancies in smallpox vaccinees
"We don't have any reason to believe that the vaccine had a causal relationship" to the miscarriages, Nowak said. HERE WE GO AGAIN. There never ever seems to be a causal relationship between vaccines and any event or disease.
Juvenile sarcoidosis after BCG vaccination
> Genevieve E. N. Osborne, MRCP
> Eleanor Mallon, MRCP [MEDLINE LOOKUP]
> Susan C. Mayou, BSc, FRCP [MEDLINE LOOKUP]
> London, United Kingdom
Abstract
We report the case of a 2-year-old boy with juvenile sarcoidosis, in whom the cutaneous lesions first arose at the site of and soon after a BCG vaccination. Juvenile sarcoidosis is rare, and the pattern of clinical features is distinct from the adult form of sarcoidosis, possibly related to immunologic development. The cause of sarcoidosis is unknown, although there is much interest in the possibility of mycobacterial species operating as antigenic stimuli to initiate the disease. This case suggests that the Mycobacterium bovis present in the BGC vaccination may have been etiologically important in the development of sarcoidosis. (J Am Acad Dermatol 2003;48:S99-102.)
This supplement is made possible through an unrestricted education grant from Stiefel Laboratories to the American Academy of Dermatology. From the Department of Dermatology, Imperial College School of Medicine, Chelsea and Westminster Hospital.
Reprint requests: Dr G. E. N. Osborne, Specialist Registrar in Dermatology, St John's Institute of Dermatology, St Thomas' Hospital, Lambeth Palace Road, London, SE1 7EH, United Kingdom.
Copyright © 2003 by the American Academy of Dermatology, Inc.
0190-9622/2003/$30.00 + 0 doi:10.1067/mjd.2003.158
http://www.reutershealth.com/archive/2003/08/08/professional/links/20030808c
lin010.html
Meningococcal C vaccine may cause relapse of nephrotic syndrome in children
Last Updated: 2003-08-08 12:33:14 -0400 (Reuters Health)
NEW YORK (Reuters Health) - Administration of meningococcal C conjugate vaccine (MCCV) appears to increase the risk of nephrotic syndrome relapse in children, according to a research letter in the August 9th issue of The Lancet.
"The risk of relapse after vaccination might be greater for this group of patients than the risk of meningococcal C infection," Dr. Richard S. Trompeter and colleagues write, "so the decision to vaccinate should be carefully considered." Immunogenic stimuli have been associated with the syndrome, but the effect of vaccination has not been investigated, the British researchers note. Their suspicions were raised when nine children with nephrotic syndrome relapsed after receiving MCCV.
Dr. Trompeter, of Great Ormond Street Children's Hospital NHS Trust in London, and colleagues investigated the relapse rate during the year before and the year after vaccination of children with steroid-sensitive nephrotic syndrome.
Included were 106 patients who received the vaccine, among whom there were 63 relapses in the year before and 96 in the year postvaccination, a relative incidence of 1.52 (p = 0.009). The risk was markedly raised only in the first 6 months after inoculation (relative incidence = 1.84). "From 106 doses of vaccine, there was a risk of one relapse in every four doses given to this population," Dr. Trompeter and colleagues write. They suggest that conjugate vaccines stimulate T cells, "so disturbance of the cytokines by MCCV might have resulted in the cluster of relapses we recorded." Lancet 2003;361:449-450.
http://www.thedailystar.net/2003/09/06/d30906011818.htm
3 babies die after 'measles vaccine'
BSS, Jamalpur
Three infants died while six others fell sick yesterday, having been injected 'measles vaccine' on Thursday at an E PI (Extended Programme of Immunisation) centre at Sabilapur under Melandah upazila. The dead, each aged about 10 months, were Mitu, daughter of Khoka Sheikh, Sumyea, daughter of Shakhawat Hossain, and Abu Bakar, son of Suja Mia of Sabilapur village.
Their bodies were sent to Jamalpur General Hospital for autopsy.
ABM Aminullah Nuri, upazila nirbahi officer (UNO) of Melandah, confirmed the death of three infants, adding that the cause of their deaths is yet to be determined. The UNO said the cause of death will be determined after autopsy. An expert team is expected to arrive here today for further investigation, he added. Meanwhile, six others -- Lima, Rupamani, Nirosha, Swapna, Sahedur and Eshrat--became sick after taking the vaccines and are undergoing treatment in different hospitals. Melandah Health Complex sources said the vaccines were made by Serum Institute of India Ltd. A case was filed with Melandah Police Station. Health Assistant Motiyar Rahman and Family Welfare Assistant Shefali Begum of the EPI centre reportedly went into hiding after the incident.
NEW DECISION ON HEPATITIS C
Wednesday, September 24, 2003
By Mike Murphy
YREKA - My office just received some really good news for veterans suffering with Hepatitis C. The newly created specialty rating team in Cleveland, Ohio known as the "Tiger Team" awarded a Vietnam veteran a service connected disability for Hepatitis C. The decision, which just came out in August of this year, was as a result of the "Jet Injectors" used for inoculations of most service members during the Vietnam Era and after.
As I have written about in previous columns, Vietnam Era veterans have been the fastest growing number of Hepatitis C patients. The biggest mystery has always been why. Many of these veterans belong to no "high risk" group such as homosexuals or IV drug users, and many did not even serve overseas. The only risk group they belong to is being in the military during this era. It appears that a link has finally been established as to the reasons for this.
A research project headed by Lawrence Deyton, MSPH, MD, the Director of Aids/Hepatitis at the United States Department of Veterans Affairs in Washington D.C. said in part, "Anyone who had inoculations with the jet injector is at risk of having Hepatitis C and should be tested." Research indicates that the Hepatitis C virus still exists on medical instruments after cleaning with many solutions. I don't believe that this statement could be any clearer.
The jet injector system has long been suspected of transmitting blood borne pathogens. In veterans groups, many believe that the VA purposely denied veterans Hepatitis C claims for being infected with this virus, to hold treatment costs down and give the VA the ability to deny the claim. There were ridiculous studies released indicating the veterans themselves were at fault due to misconduct in, or after military service, that justified the denials.
I remember, not too long ago, the Agent Orange issue was a similar denial by the government and so was the "Gulf War Syndrome." There were similar ridiculous studies released indicating that there was no proof that Agent Orange made anyone ill. Now we all know better. The government went so far as to state that the "Gulf War Syndrome" was a psychosomatic disorder and did not really exist. Now we know better. And now finally we see the truth regarding Hepatitis C. It would appear that the Emperor has no clothes.
The biggest problem to overcome in this issue is getting the word out to these veterans. Most of us who served during this era can remember the long inoculation lines and blood running freely down many of our arms during these inoculations with the jet injectors. Another problem is that the incubation period for Hepatitis C can be decades long and symptoms may be nonexistent up until the time that the veteran becomes very ill. I am asking that anyone who reads this column, please, spread the word and get tested. You can be tested at any VA facility. If you don't know how to access the VA, call our office in Yreka and we will help you. This is extremely important. Your life and the lives of your loved ones may depend on it.
http://www.eastandard.net/headlines/news05102003003.htm
Twins die after polio vaccine
By Joseph Murimi
Four-month-old twins died on Friday night shortly after they were immunised against polio in Kinangop in Nyandarua district. The twins — James Maina Mburu and Samuel Mwangi Mburu — had earlier in the day been taken to Nandaras Health Centre where they were given the oral vaccine. The twins were not sick when they were taken to the health facility for the routine immunisation by their 30-year-old mother, Scola Muthoni Mburu. Muthoni said that when she returned to her home at the Nandaras Scheme, the children did not show any signs of sickness even as late as 8 pm.
At about 1 am, Muthoni said, she woke up and breastfed them, then went back to sleep. She did not notice anything amiss. However, when she woke up yesterday morning at about 8 am, she was shocked to find both twins dead. She called in neighbours and police. It was suspected the polio dose and other injections that go with it may have caused the death. Their bodies are at the Naivasha District Hospital mortuary where a post-mortem will ascertain the cause of death.
Vaccine Blunder Leaves Hundreds Risking Diseases"
Glasgow Evening Times (Scotland) (www.eveningtimes.co.uk) (11/20/03) P. 6;
Anderson, Deborah
The National Health Service in Lanarkshire, Scotland, is contacting over 200 patients after discovering that a number of vaccinations given at Clarkston Medical Centre used vaccines that had passed their expiration dates. The vaccinations may not provide enough immunity, and a phone help line has been set up for those who think they may have received one of the injections. "We can understand that people will be concerned but can assure them that the risk to their health is minimal," says Public Health Medicine Consultant Dr. David Cromie. Some of the vaccinations may have been given over two years ago, and the vaccines given include all childhood vaccinations as well as tetanus and polio vaccines for adults.
http://news.telegraph.co.uk/news/main.jhtml?view=DETAILS&xml=/news/2003/11/2
1/nvacc21.xml
200 patients are given out-of-date vaccines
By Auslan Cramb, Scottish Correspondent
(Filed: 21/11/2003)
More than 200 people have been immunised with vaccine that was past its expiry date, it was disclosed yesterday. The doctor who made the error also admitted that the vaccines, administered over the past two years, had been stored at the incorrect temperature. Dr Tahira Idrees made the errors at the Clarkston Medical Centre, Airdrie. NHS Lanarkshire said some or all of the vaccines might not have given the correct level of immunity. The vaccines were given to patients mainly for childhood diseases such as polio, diphtheria, tetanus, meningitis C and MMR (measles, mumps and rubella).
The health trust is contacting all patients but said the risk to health was minimal. Dr David Cromie, a consultant in public health medicine, said patients would be invited to discuss their concerns and to attend appointments to receive new vaccinations. "We have prioritized the cases involving children up to seven years old," he said. "There have been no reported cases of childhood illness due to ineffective vaccine. "We are treating this situation seriously and a thorough investigation of the circumstances is under way. "The GP concerned has expressed her regret at the anxiety and inconvenience caused."
Also Monday, Kaiser Permanente of Colorado said more than 50 people may have inadvertently been given a polio or pneumonia vaccine instead of the flu shot. Six people may have been given polio vaccine in Englewood and 50 may have gotten a pneumonia shot in Lakewood, spokeswoman Jacque Montgomery said. Neither vaccine should cause health problems and everyone involved will be offered the flu shot again, she said.
http://www.local6.com/news/2665918/detail.html
http://www.sciencedaily.com/releases/2003/12/031223062104.htm
2003-12-25
Invalid Vaccine Doses Would Cost Millions To Fix
Children who receive some of their vaccine doses too soon may need to be revaccinated, at an extra cost of $10 to $18 million a year, according to a new study. "The cost of revaccinating these children is substantial and may impact parents, physicians and vaccine purchasers," say Shannon Stokley, M.P.H. of the Centers for Disease Control and Prevention and colleagues. Their findings appear in the January issue of the American Journal of Preventive Medicine.
Stokley and colleagues' sample of national immunization records reveals that 10 percent of children received at least one invalid vaccine dose in 2002. Invalid vaccines are doses administered five or more days before the minimum age for the first dose or before the minimum time between doses has elapsed. Invalid doses need to be repeated to ensure that children are adequately protected from disease, according to the researchers.
"It is important that vaccines be administered at an age when a child can develop a proper immune response and before significant exposure to natural infection," Stokley says. Of the 2002 incorrect doses, half were for the hepatitis B vaccine, 19 percent were for diphtheria-tetanus-pertussis, 15 percent for chicken pox, 12 percent for measles and only 4 percent for polio.
The first and final doses in a vaccine series were the ones most likely to be given too early, the researchers found. For instance, all of the incorrect hepatitis B doses were the the third and final dose. First doses made up 98 percent of all invalid polio vaccinations. Children who received vaccinations from multiple healthcare providers and those who were born outside the United States were more likely to get an invalid dose. American Indian, Hispanic and Asian children were also significantly more likely to get an invalid vaccination.
Children with mothers who had completed at least some college were less likely to get incorrect doses than those with mothers who had not completed high school, Stokley and colleagues concluded. The study included immunization data collected from 34,087 parents and 22,958 physicians for children ages 19 to 35 months. This story has been adapted from a news release issued by Center For The Advancement Of Health.
Subject: EU drug watchdog probes Aventis, Glaxo vaccines
http://www.signonsandiego.com/news/
business/biotech/20031202-0529-health-europe-vaccines.html
EU drug watchdog probes Aventis, Glaxo vaccines
window.onerror=function(){clickURL=document.location.href;return true;}
if(!self.clickURL) clickURL=parent.location.href; var js=0.0;js=1.0;js=
1.1;js=1.2;js=1.3;js=1.4;js=1.5;
REUTERS
5:29 a.m. December 2, 2003
LONDON – The European Medicines Evaluation Agency will start an active surveillance programme of so-called hexavalent vaccines early next year following reports of a small number of sudden unexpected deaths in vaccinated children. In a statement on its Web site, the agency said four two-year-olds had died soon after being vaccinated, following the authorisation of Hexavac from Aventis-Pasteur MSD, a joint venture of Aventis SA and Merck & Co Inc, and Infanrix Hexa, made by GlaxoSmithKline Plc, in 2000. These vaccines offer millions of children protection against six life-threatening infectious diseases – diphtheria, tetanus, poliomyelitis, whooping cough, hepatitis B and Haemophilus influenzae b. The agency said statistical analysis on data from Germany, where three children died among more than 700,000 given a booster jab, showed that the number of deaths within 48 hours of Hexavac administration exceeded the expected number.
Experts on the agency's Committee for Proprietary Medicinal Products considered that this raised possible suspicions, though they stressed there was no plausible biological reason to link the deaths with vaccination. However, to find out more, prospective and retrospective studies would be carried out by independent institutions and assessed by regulatory bodies. "The results will be closely monitored so that timely regulatory action can be taken, if necessary," the agency said. In the meantime, it reaffirmed its view that the benefits of vaccination far outweighed the possible risks of existing vaccines and that vaccination should be continued according to national vaccination schedules.
Some Arizonans may have gotten ineffective flu shots
Kerry Fehr-Snyder
The Arizona Republic
Jan. 2, 2004 05:30 PM
More than 20 residents in Maricopa County may have received old or ineffective flu vaccines from an unlicensed provider being investigated by prosecutors in Washington. Another dozen residents in Yavapai County also received questionable vaccines from MedSources Inc., a company owned by Shahid Sheikh, prosecuters said Friday. Sheikh is a registered counselor, not a doctor, and is not authorized to give injections, they said. MedSources also gave flu shots in Nevada, Idaho and possibly California, a spokesman for the Bellevue, Wash. Police Department said. "This kind of thing is a rare aberration," said Dr. Bob England epidemiologist with the Arizona Department of Health Services. "It demonstrates that people will try to run scams when they can."
The state health department provided county health departments in Maricopa and Yavapai with a list of individuals who received flu vaccines from MedSources. Those counties are notifying individuals that their vaccines came from an unauthorized provider, and advising them to check with their doctors about receiving another flu vaccine, especially if they are at high risk for complications from the flu.. "Most people are just taking it in stride," said Doug Hauth, spokesman for the Maricopa County Department of Public Health Serivces.
Investigators believe that MedSources gave consumers outdated or weakened vaccines after one of its workers was told to dilute doses, investigators said. They also found Sheikh had flu vaccine made for last flu season that is ineffective against current flu strains. MedSources gave the vaccines at worksites to about 700 individuals, including at least 31 in Arizona. England said he's not aware of any test to determine whether an individual's flu shot will provide protection against the flu. The state health department says about 500 doses of flu vaccine are available in Arizona. Information:
www.cir.org, 602-263-8856 or 1-800-352-3792.
This article can be found at:
http://www.azcentral.com/news/articles/0102badvaccine02-ON.html
http://news.bbc.co.uk/1/hi/scotland/3352425.stm
Inquiry after error over baby MMR
An investigation has been launched after a baby was accidentally given the controversial MMR injection. The three-month-old girl was supposed to have received a meningitis jab. It is understood the mistake was made at Gorbals Health Centre in Glasgow. A spokeswoman for NHS Greater Glasgow confirmed an apology had been issued to the family. Guidelines state babies should be at least 13 months old before they get the measles, mumps and rubella vaccine.
Public health experts said the incident should not cause any long-term problems for the child. Politicians described the mistake as appalling. Scottish Socialist MSP Tommy Sheridan said: "I was horrified when I heard. This case again highlights the issue of the triple MMR vaccine." Scottish National Party health spokeswoman Shona Robison said that "lessons must be learned" from the mistake.
MMR shots go wrong
By Richard Simcox
WORRIED parents have been told their babies’ MMR jabs could be ineffective after being stored at the wrong temperature. Dozens of mums and dads have been recalled to three clinics after the mix-up affecting several vaccinations. The controversial combined measles, mumps and rubella vaccination has been at the centre of a health scare over possible links with autism.
Now parents fear giving their child a second dose so shortly after the first could be dangerous. Tracey Keyes received a letter from director of public health Dr Angela Bhan saying it “could mean” her 17-month-old son Ben’s MMR vaccination was not fully effective. The 29-year-old, who lives in Mosslea Road, Bromley, with husband, Paul, 35, said: “We were appalled they should tell us this without any offer of testing to see whether the first dose had worked. “Given the current controversy surrounding MMR, this was vague at best. There are endless questions we would like answered before we even consider giving our son another vaccination.” Mrs Keyes said she had been persuaded by government advice not to pay £150 to have the single jabs separately at a private clinic.
In her letter to parents, Dr Bhan apologised for any “anxiety or distress” caused. She wrote: “We are sorry this has happened but want to assure you there is no risk of any harm caused by the vaccination your child received.
“To make sure your child is fully protected, we strongly recommend you come back to the clinic to have your child vaccinated again.” In all, 68 children, given either polio, MMR, whooping cough or haemophilus influenzae b vaccinations at Princes Plain, Biggin Hill and Bromley North clinics have been recalled. Nine received the MMR jab.
Bromley Primary Care Trust, which runs the clinics, says it will wait at least one month before re-vaccinating. Dr Nada Lemic, also a director of public health for the PCT, said: “We have reassured parents there is no risk of harm and no cause for concern. “Repeating the vaccination after one month is safe and evidence exists to show adverse reactions to MMR — such as fever, rash or headache — are fewer after a second dose. “We very much regret the inconvenience and worry this may have caused.” The PCT has replaced the faulty refrigerators, checked all the other clinics in the borough and upped the frequency of routine temperature checks. Parents can call the public health office on 01689 880687 during office hours for more information.
4:22pm Tuesday 26th August 2003
http://www.cnn.com/2004/HEALTH/02/13/smallpox.reut/index.html
Breast-fed baby exposed to smallpox vaccine virus
Friday, February 13, 2004 Posted: 9:08 AM EST (1408 GMT)
ATLANTA, Georgia (Reuters) -- A U.S. soldier's wife who was accidentally exposed to the live virus in the smallpox vaccine likely passed it on to her baby through breast-feeding, according to
military authorities.
The incident, which occurred in May 2003 as the U.S. military was inoculating hundreds of thousands of soldiers against smallpox, is the first documented case of third-hand transmission of vaccinia virus through breast-feeding. Vaccinia is not smallpox, but a pox-like virus related to the rare and deadly virus. Cases of accidental transmission are rare, usually occurring through direct contact with a vaccinated person's unhealed vaccination site.
In a report released on Thursday by the U.S. Centers for Disease Control and Prevention, a team of Army doctors reported that the unidentified mother developed lesions near her nipples about a week after her husband was vaccinated. Although the husband had a "major reaction" to the jab, the couple had continued to sleep together and the wife had not stopped breast- feeding their baby. About two weeks later, lesions appeared on the infant's face and tongue. Lab tests confirmed that mother and daughter had been exposed to vaccinia. Neither had been vaccinated for smallpox.
Military officials are not exactly sure how the wife, who had handled laundry possibly contaminated with vaccinia, was infected. They are, however, fairly certain that the baby got the virus through breast- feeding. The report urged breast-feeding mothers living with people who have been vaccinated against smallpox to be aware of the possibility of exposure to vaccinia and take care not to spread it to their infants.
The Department of Defense, which has confirmed 18 cases of inadvertent vaccinia transmission since December 2002, has been advising vaccinated persons to cover up their vaccination sites, wash their hands regularly and limit contact with infants. The United States ended routine vaccinations for smallpox in 1972, but decided in 2002 to resume them for soldiers and some health-care workers as fears grew that the virus could be used as a weapon by radical groups or countries like Iraq.
Smallpox kills about 30 percent of its victims and scars the remainder for life.
http://abcnews.go.com/wire/World/ap20040224_1427.html
Vaccine Boycott Grows in Northern Nigeria
KADUNA, Nigeria Feb. 24 — Two more states in Nigeria's Islamic north joined a boycott Tuesday of a massive polio immunization campaign, demanding government proof the vaccines don't spread AIDS or sterility as Islamic leaders contend. Nigeria, meanwhile, announced it would be days before it had results of lab tests meant to prove the vaccine's safety too late to change any minds among Muslim leaders in the four states that are blocking the immunization campaign, due to end Thursday.
"This is an opportunity lost," U.N. Children's Fund spokesman Gerrit Beger said, confirming that Niger and Bauchi states had joined the vaccine boycott pending findings of the government-led investigation. Northern Nigeria Islamic leaders say the immunization campaign is part of a U.S. plot to depopulate Muslim northern Nigeria by spreading AIDS or sterilizing agents. Northern states maintain their own lab tests show contaminants in the vaccine.
Fourteen million people live in the four states that have blocked the immunization. The World Health Organization says a polio outbreak spreading from one of the states, Kano, has helped spread polio to seven African nations where it had been eradicated. The outbreak and vaccine ban threaten a 16-year worldwide effort to wipe out polio globally by 2005, WHO says. WHO launched the 10-nation emergency immunization campaign on Monday, sending hundreds of thousands of volunteers door-to-door with vaccine to inoculate 63 million children.
On Tuesday, some Muslim families turned away vaccination teams even in states where the campaign has been allowed. Complicating matters, Nigerian Muslim tradition bans male strangers from entering homes with women and girls, forcing the medical teams to send in girls as young as 14 to carry out the inoculations.
"It is difficult to train these young girls to communicate effectively with parents and vaccinate all children in a methodical way without missing some areas," said Usman Kariko Binawa, a vaccine campaign organizer. Just outside the northern city of Kaduna, in the predominantly Muslim village of Maraban Jos, health workers scrawled check marks with chalk on the mud and tin houses of families accepting vaccinations. They wrote large "R's" on the those who refused, so they wouldn't be bothered again. Binta Abdullahi, a 34-year-old campaign worker in Maraban Jos, came out of the home of one Muslim family that had refused to have their children immunized.
"They want to wait for the results of the examinations," she said, referring to the government investigation. In the nearby village of Barakal Allahu, which is mostly Christian, residents welcomed the teams. "We are eager. We've seen polio and we don't want it for our children," said Stephen Dogo, 74, after his two youngest children were vaccinated. In the same village, one polio victim a young wheelchair-bound man with twisted, skinny legs rode past health workers, expressing his approval with the words "God is Great."
Earlier this month, the Nigerian government sent politicians, scientists and religious leaders abroad to witness the polio vaccine been tested in foreign labs. On Tuesday, the fact-finding team said in a statement it was awaiting test results from labs in India and expected them as late as the end of the month, after the vaccine campaign is over. The Associated Press obtained a copy of the committee's interim report that ruled the vaccines safe. However, it acknowledged the tests showed "trace amounts of estradiol," a form of the female hormone estrogen the vaccine's Muslim detractors claim could cause infertility.
The unsigned four-page document suggested the hormone may have come from calf blood serum it said was sometimes used to help produce the vaccine. WHO officials have repeatedly insisted minute amounts of hormones would be completely harmless, amounting to less than what is found in human breast milk. Nigerian Health Ministry officials could not immediately be reached for comment on the leaked document. Muslims in Nigeria's north have been wary of vaccine campaigns since 1996, when families in Kano state accused New York-based Pfizer Inc. of using an experimental meningitis drug without fully informing of the risks.
The company denied any wrongdoing. A U.S. court dismissed a lawsuit by 20 disabled Nigerians who allegedly took part in the study, but a U.S. appeals court later revived it.
Notice to Readers: Manufacturer's Recall of Human Rabies Vaccine --- April 2, 2004
CDC and the Food and Drug Administration (FDA) have been notified that a recent quality-assurance test of IMOVAX® Rabies Vaccine (Aventis Pasteur, Swiftwater, Pennsylvania) identified the presence of noninactivated Pitman-Moore virus (the attenuated vaccine strain) in a single product lot. IMOVAX® is an inactivated viral vaccine and should not contain live virus. The vaccine lot containing noninactivated virus was not distributed.
As a precautionary measure, Aventis Pasteur initiated a voluntary recall of lot numbers X0667-2, X0667-3, W1419-2, and W1419-3, which were produced during the same period as the lot that contained noninactivated Pitman-Moore virus. These four lots, which were distributed in the United States from September 23, 2003 through April 2, 2004, passed all FDA-approved release tests, including testing to confirm the absence of live virus. These test results suggest that any potential risk to those vaccinated with recalled vaccine is likely to be low. No unusual adverse events associated with the recalled vaccine have been reported.
The manufacturer has indicated that additional lots of recalled vaccine were distributed internationally. These lots also passed all release tests, including testing to confirm the absence of live virus. The manufacturer is working with regulatory authorities to determine lot numbers of vaccine and countries that might have received recalled lots. More information about these internationally distributed lots will be provided as it becomes available.
Aventis Pasteur is providing additional detailed information to all distributors and providers. Health-care providers should contact persons who received recalled vaccine to implement the recommendations outlined in this notice (see Recommendations for Persons Who Received Recalled Vaccine). In addition, persons who know they received rabies vaccine between September 23, 2003, and April 2, 2004, should contact their health-care providers to determine whether they received vaccine from one of the four lots being recalled and, if so, whether they should be treated as outlined below. Vaccine distributors and health-care providers who have any remaining doses of the recalled lots should not use them and should contact Aventis Pasteur regarding their disposition. Information about this recall is available from the Aventis Pasteur Medical Information Services Department, telephone 800-835-3587, or at http://www.vaccineshoppe.com.
All persons who have begun a rabies vaccination series (whether for pre- or postexposure prophylaxis) must complete that vaccination series on time, using nonrecalled vaccine. Information about human rabies prevention based on current recommendations of the Advisory Committee on Immunization Practices (ACIP) is available at
http://www.cdc.gov/mmwr/preview/mmwrhtml/00056176.htm.
Recommendations for Persons Who Received Recalled Rabies Vaccine
Most persons receiving rabies vaccine do so because of exposure to a rabid animal, and treatment is needed to prevent fatal illness. Thus, persons who are receiving postexposure prophylaxis (PEP) must not omit or delay receiving any remaining injections; injections needed to complete the series should use nonrecalled vaccine. Recalled vaccine is considered fully immunogenic, and previously administered doses can be considered a dose in a PEP regimen.
Although unlikely, a theoretical possibility exists that persons who received vaccine from a recalled lot could have been exposed to the noninactivated Pitman-Moore vaccine strain of rabies virus. Even in the event of such an exposure, the timely administration of treatment, as described here, will help to ensure negligible risk to persons who have received vaccine from a recalled lot. Persons who received recalled vaccine should receive treatment equivalent to PEP, similar to published guidelines, as follows:
Persons who were vaccinated with recalled vaccine as part of a course of PEP for a possible rabies exposure. Not previously immune (i.e., persons who had not received at least 3 doses of vaccine at some time before the possible rabies exposure). Persons without prior immunity who have a possible rabies exposure routinely receive a 5-dose postexposure immunization series. If this postexposure series has not already been completed, such persons should complete the full postexposure series, using nonrecalled vaccine to complete the series. Doses that have been administered already as part of the 5-dose series need not be repeated, even if recalled vaccine was used. In addition, if rabies immune globulin (RIG)* was not administered with the first dose of vaccine and it has been <7 days since the first dose of vaccine, RIG should be administered at this time. Once PEP is completed, persons are considered fully vaccinated against both the original rabies exposure and any possible exposure to noninactivated virus in the recalled vaccine.
Previously immune (i.e., persons who had received at least 3 doses of vaccine at some time before the possible rabies exposure). Persons with preexisting immunity (i.e., who have completed a full preexposure or postexposure vaccination series) who then have a possible rabies exposure routinely receive 2 booster doses of rabies vaccine. If one or both doses already were administered using recalled vaccine, such persons should receive 2 more doses, using nonrecalled vaccine. RIG is not recommended. Persons who were vaccinated with recalled vaccine for reasons other than a possible rabies exposure.
Not previously immune (i.e., persons who had not received at least 3 doses of vaccine at some previous time). Persons without prior immunity who received recalled vaccine as part of a 3-dose preexposure vaccination series should receive additional doses using nonrecalled vaccine for a total of 5 doses (dosing intervals should follow the PEP schedule as closely as possible). RIG* is recommended if <7 days have elapsed since administration of the first dose of vaccine. Previously immune (i.e., persons who had received at least 3 doses of vaccine at some previous time). Persons with preexisting immunity (i.e., who have completed a full preexposure or postexposure vaccination series before they received recalled vaccine) who received recalled vaccine as a routine booster dose should receive 2 additional doses of nonrecalled vaccine. RIG is not recommended.
All clinically significant adverse events following receipt of rabies vaccine should be reported to 1) Aventis Pasteur, telephone 800-835-3587 and 2) the Vaccine Adverse Event Reporting System (VAERS) at http://www.vaers.org, or telephone 800-822-7967. Additional information about rabies and its prevention is available from CDC, telephone 404-639-1050, or at http://www.cdc.gov/ncidod/dvrd/rabies.
[PROVE Note: People taking a rabies vaccine now have to worry about being exposed to the fatal live rabies virus. In the manufacturing process, a filter failed. Next time somebody tries to tell you the flu vaccine can't give you the flu, or the IPV vaccine (inactivated polio vaccine) can't give you polio, tell them mistakes happen in manufacturing and send them this one.]
http://www.cnn.com/2004/HEALTH/04/03/vaccine.recall/index.html
Thousands of doses of rabies vaccine recalled
Drug firm: One lot had live virus; 3 called back as precaution
(CNN) -- A vaccine maker is recalling thousands of doses of rabies vaccine after discovering through routine testing that one lot contained live virus that could potentially harm human health.
The lot had not been distributed, Aventis Pasteur spokesman Len Lavenda said in a telephone interview with CNN from the company's U.S. headquarters in Swiftwater, Pennsylvania.
As a precaution, Aventis Pasteur is also recalling three other batches of Imovax that were made at the same time, the Lyon, France-based company said.
The Centers for Disease Control and Prevention said in a statement that the lots being recalled were distributed between September 23 and April 2, and "had passed all FDA-approved release tests, including testing to confirm the absence of live virus," suggesting the risk to anyone who may have received the vaccine is small.
Still, anyone who received rabies vaccine between those dates should contact their health care provider, the CDC dispatch said. No unusual events associated with the recalled vaccine have been reported, it added.
Rabies vaccine is made by killing live rabies virus. Injections of the dead virus stimulate the body's immune system to develop antibodies that kill the live virus.
That process went awry at the company's plant in Lyon, where a bulk vaccine filter failed, Lavenda said. That's a critical step in the production process because the solution that typically kills isolated particles of the live virus may not inactivate large particles of it, he said, adding that the filter has been fixed.
Human rabies is rare in the United States, with only 39 cases diagnosed since 1990. But doctors treat possible exposures aggressively because once symptoms develop, the disease is almost always fatal.
Two groups of people get rabies vaccines: those who have been bitten by an animal that is rabid or suspected of being rabid; and those at risk of being bitten, such as veterinarians, zookeepers and people traveling to regions where rabies is common.
Last year, in the United States, about 18,000 people at risk of being bitten got preventive vaccinations, and another 40,000 got them after being bitten.
The only people who need to do something as a result of the recall are those who received the vaccine preventively, Lavenda said. The others, in addition to getting the vaccine, would have been given immune globulin, which contains antibodies that kill live virus. Aventis will pay for those people to get an injection of immune globulin, he said.
The company is also recalling vaccines that were distributed to 18 other countries, and will be sending its customers information about the recall on Monday, Lavenda said.
http://news.bbc.co.uk/1/hi/england/west_yorkshire/3732523.stm
Apology over 'unwanted' MMR jab
Health chiefs have apologised to the parents of a young girl who was given the MMR jab against their wishes. Three-year-old Stevie Hall received the injection at her nursery school in Leeds - even though her parents signed two forms refusing the vaccine. Tim and Jane Hall are now considering taking legal action against Leeds West Primary Care Trust (PCT).
A spokeswoman for the PCT confirmed the incident, which they said was down to a "regrettable mistake".
Human error
Lilian Dalton, business support manager for the PCT, said: "Leeds West Primary Care Trust is sorry if this incident has caused Mr and Mrs Hall upset or distress. "The PCT believes in patient choice, including choice of immunisation. "However, in this instance it would appear that a member of our front line staff made an honest, yet regrettable, mistake and Mr and Mrs Hall's daughter was given the immunisation without parental consent. "We are currently reviewing our procedures to see if changes need to be made in order to avoid mistakes of this nature in the future."
Stevie was given the jab by a health worker who visited Whingate School in Armley, Leeds, on Monday 17 May. Her parents had signed a second form refusing their consent for the vaccination after the original was misplaced.
MMR controversy
They told the Yorkshire Evening Post they signed a form for polio and Hib vaccines, but said no to the combined measles, mumps and rubella injection. Mr Hall, 43, of Armley, said he did not want to take the risk of his daughter receiving the jab because of his uncertainty over the risks involved. He said he was "livid" after hearing about the mistake. The controversy about the MMR injection has raged since 1998 when The Lancet published research that raised the possibility of a link to autism.
Ten of the doctors who co-authored the study, which never proved a link, have since said there was insufficient evidence to draw that conclusion.
Don't they have a lot of these *accidents*?
~~~~~~~~~~~~~~~~~~~~
http://www.northamptontoday.co.uk/ViewArticle.aspx?SectionI
D=255&ArticleID=798768
Boy gets MMR jab without permission
A YOUNG boy was given an MMR injection at a Daventry medical practice even though his parents had refused the controversial jab. The mother of four-year-old Jack Farmer is angry at the mix-up, for which doctors at the Abbey House Medical Practice have issued an "unreserved" apology. Julia Farmer, of Horncastle Close in Daventry, took Jack to the surgery for injections for diphtheria, tetanus, whooping cough and polio, but he was also given an MMR booster without her consent.
Mrs Farmer, 36, said: "I checked Jack's medical book when we left the surgery and realised he had been given the MMR. They were very apologetic but it's something that can't be undone now." A single MMR jab to immunise against measles, mumps and rubella has been linked, without proof, to the development of autism in children. Like many parents, Mrs Farmer and her husband Angus chose to pay for three separate immunisation jabs for four-year-old Jack and daughter Alice, two. Individual jabs are claimed to reduce the autism risk.
Mrs Farmer said: "I am a nurse and my husband and I made an informed decision about how our children would receive the vaccines. "When something is put into your child you haven't given consent for, it's wrong." Abbey House Medical Practice manager Nigel Brown confirmed a child was given the MMR vaccination without the consent of parents. He added: "This incident is viewed most seriously by the practice and a full investigation is being carried out. "The practice again would like to apologise unreservedly to the family for the incident and new procedures will be introduced to prevent a recurrence of the events."
alyson.fixter@northantsnews.co.uk
An outbreak of hepatitis B associated with jet injections in a weight reduction clinic.
Canter J, Mackey K, Good LS, Roberto RR, Chin J, Bond WW, Alter MJ, Horan JM.
Division of Field Services, Centers for Disease Control, Atlanta, GA 30333.
From January 1984 through November 1985, 31 clinical cases of hepatitis B occurred among attendees of a weight reduction clinic (clinic 1). Before the onset of illness, each case-patient had received a series of injections of human chorionic gonadotropin administered by jet injectors at clinic 1. Clinical history, risk factor assessment, serologic evaluation, and review of clinic injection records were obtained on 287 (84%) of 341 persons who had attended clinic 1 in the first 6 months of 1985. Of this cohort, 21% (60/287) had evidence of acute infection with hepatitis B virus (either documented clinical cases or antibody to hepatitis B core antigen, IgM positive). Of persons who had been given human chorionic gonadotropin at the clinic during the period studied, 24% (57/239) of those receiving human chorionic gonadotropin only by jet injector experienced acute hepatitis B virus infection. None of the 22 persons who had received injections only by syringe experienced hepatitis B virus infection. Stopping the use of the jet injectors on July 2, 1985, at clinic 1, was associated with the termination of this outbreak. This investigation demonstrated that jet injectors can become contaminated with hepatitis B virus and then may be vehicles for its transmission.
PMID: 2393323 [PubMed - indexed for MEDLINE]
http://news.scotsman.com/latest.cfm?id=3031039
Doctor Sacked for Giving Wrong Vaccine to Child
By Martha Linden, PA News
A £90,000-a-year doctor was sacked from his job at a private clinic after he gave a child the wrong vaccine and then attempted to cover up his tracks, a disciplinary hearing was told.
Dr Michael Wetzler, of Berkhamsted, Herts, admitted administering the rubella vaccine instead of the measles vaccine to 23-month-old Bradley Wright when the youngster was brought to him last year at the Breakspear Medical Group, in Hemel Hempstead.
At a hearing of the General Medical Council, Dr Wetzler admitted that once he became aware that he had administered the wrong vaccine, he misled Bradley’s parents, leaving them to believe that he had given the measles dose.
He further admitted falsifying the child’s immunisation records by crossing out the word ‘rubella’ and substituting ‘measles’, and providing the boy’s parents with a leaflet relating to the measles vaccine.
The hearing was told Bradley was brought to the clinic, which specialises in allergies and environmental disorders, on June 20 last year by his parents for a measles immunisation.
Adrian Darbishire, for the GMC, said Dr Wetzler had been 40 minutes late for his appointment with the Wright family. He said the couple’s impression of Dr Wetzler had been one of “rushing through the injection”.
Following the immunisation, Mrs Wright felt unhappy about the way Dr Wetzler had treated them, the committee was told.
They became suspicious that their child had been given the wrong vaccine after consulting Bradley’s child health record and noticing that an entry had been made and then crossed out, the committee heard.
Mr Darbishire said Mr Wright asked Dr Wetzler what he had given Bradley. Dr Wetzler admitted that he had given him rubella in error.
Dr Wetzler later appeared before the directors of the clinic and was interviewed about the incident, the committee was told.
In the interview he admitted lying to the Wrights and falsifying documents, and was dismissed for gross misconduct.
Mr Darbishire said Dr Wetzler had told the bosses he had been asked to do “demeaning work” at the clinic.
Earlier, the committee had been told that Dr Wetzler had been appointed in March last year by the clinic with a view to playing a senior role at the establishment.
He was known to the clinic because of his work with cancer patients in Bristol in the early 1980s. He had applied for a job with them from South Africa, where he had worked for a number of years.
The committee heard that Mrs Wright had been “incredibly surprised” to receive a phone call at home from Dr Wetzler after he was dismissed, in which he had told her he had been “very fed up” on the day he had seen them with their son.
He had been refused leave and had been doing “back-to-back vaccinations” of single-dose MMR jabs at the clinic. He told Mrs Wright he had acted in an “immature manner” in the way he had dealt with the error, but he had intended to put it right at the next appointment.
At that point the doctor had become “emotional and started to cry”, Mr Darbishire said.
In further correspondence with the clinic bosses, Dr Wetzler wrote that he felt he would “die of repetitive needle disease” as a result of administering single-dose MMR vaccines to the children of parents anxious about the possible effects of the combined jab.
He said he hoped to bring his deeper, personal expertise to the post when he joined the Breakspear clinic, but instead had been forced to take part in the “mind-numbing activity” of administering the single doses.
Mr Darbishire said there was no suspicion of any harm being caused to the young patient by Dr Wetzler’s action, and he also noted the doctor’s immediate admission of what he had done when he was confronted by Mr Wright at the clinic.
But he said if Bradley had suffered an adverse reaction then medical treatment would have proceeded on an “false basis”.
The hearing continues.
Researchers accidentally exposed to live anthrax
By PAUL ELIAS
AP Biotechnology Writer
SAN FRANCISCO At least five workers developing an anthrax vaccine at a children's hospital research lab in Oakland were exposed to the deadly bacterium because of a shipping foul up, officials reported Thursday. Officials with the Children's Hospital Oakland Research Institute said none of the researchers have shown symptoms of infection, but are being treated with antibiotics as a precaution.
Hospital spokeswoman Bev Mikalonis said the researchers believed they were working with a dead version of the anthrax bacterium, but were instead shipped live anthrax by the Frederick, Md. lab of the Southern Research Institute, which is headquartered in Birmingham, Ala. Mikalonis said other workers may have also been exposed while the live anthrax was being handled and that federal, state and local officials - including the FBI - are investigating.
The hospital doesn't believe that anyone was infected because researchers used proper procedures and precautions while handling the anthrax shots. The Oakland lab itself is located about one mile from the Children's Hospital and Mikalonis said exposure doesn't pose a threat to the hospital and surrounding community. The researchers are working with dead bacterium to develop an anthrax vaccine that children can use. Anthrax attacks killed five people and sickened 17 others in 2001 in the United States. No one has ever been arrested for the killings, but the attacks spurred development of better vaccines and treatments for anthrax than are currently available.
Mikalonis said the Oakland researchers received and stored a shipment of what they thought to be dead anthrax from the Southern Research Institute about three months ago. Last week, the Oakland researchers began injecting anthrax from the shipment in mice as part of an experiment. On Monday the mice unexpectedly began to die. On Wednesday night, California state health officials confirmed their worst fears: live anthrax was in the syringes. Agents with FBI's bioterrorism unit removed the samples from the lab Wednesday.
Southern Research Institute's Thomas Voss, who is in charge of homeland security and emerging infectious disease, said the nonprofit company is investigating what happened. Voss said it's still unclear whether the institute did ship live anthrax to Oakland. "We aren't totally sure of the sequence of events," Voss said. The Southern Research Institute was founded in 1942 and has two highly secure "hot labs" that store some of the world's most deadliest diseases. Labs and researchers from around the country that need data about those nasty diseases but don't - or can't - handle them contract with the Southern Research Institute to do that work.
Voss said the institute's hot labs in Frederick and Birmingham handle just about every "select agent" listed with the Centers for Disease Control and Prevention in Atlanta. The institute is one of 350 entities registered to handle live anthrax with the CDC. It employs 600 people nationwide and has about $75 million in revenue a year, Voss said. Voss said that while the institute receives many shipments of live diseases, it rarely ships them out. "We receive agents on a routine basis," Voss said. "But on our end, we ship very infrequently. I can't even recall shipping live agents."
The mishap will surely be seized on by critics of the government's effort to combat biological terrorism by paying for the construction or expansion of 18 high-containment labs across the country. Supporters of the building boom said the additional lab space is needed to combat emerging global threats, but critics said such expansion increases the likelihood of accidents like the one Oakland reported Thursday.
From 1998
http://www.ias.org.nz/children_contract_tuberculosis.htm
133 Children Contract Tuberculosis in Southern Kazakhstan Itar Wire Service (02/05/98) Kazakhstan health officials report that 133 children in the city of Zhanatas contracted tuberculosis during a routine fall vaccination last year. Although the children contracted TB as early as last September, local authorities reportedly concealed the incident from national officials as they tried to cure the children on their own.
According to deputy chief of Kazakhstan's sanitary and epidemiological surveillance service Albert Askarov, however, their inability to do so forced them to report the incident several days ago. Askarov said the children were infected because untrained medics accidentally used the wrong, more concentrated formulation of the vaccine for injections.
National and regional health officials are now investigating the situation.
Daily Star
Committed to PEOPLE'S RIGHT TO KNOW
Vol. 5 Num 33 Mon. June 28, 2004
Child dies after vaccination in Khulna
Staff Correspondent
A nine-month-old baby girl in Paikgachha upazila of Khulna district died Thursday morning after vaccination at a public immunisation camp. Our Khulna correspondent said Shahanara of Gopalpur village was taken for diphtheria, tetanus and pertussis (DTP) jab to the camp under Extended Programme of Immunisation (EPI).
The vaccine, under routine EPI programme, was the child's second dose in the last three months and seventh dose from the same vial of the vaccine that has also been put into six other children at the camp.
The health directorate described the death as 'unfortunate' and said no one is to be blamed for the incident.
According to the family sources, Shahanara went into severe convulsions on her way home soon after she received the intra-muscular injection vaccine. As she vomited and her condition deteriorated fast, her parents took her to the local upazila health complex. The attending doctor there declared her dead on arrival.
Sources said Civil Surgeon Dr Hame Jamal visited Paikgachha shortly after the incident and asked the father of the child, Punai Gazi, to bury the girl as quickly as possible without an autopsy. Punai, a day-labourer, was also paid some money to compensate for the two days he has had off work due to the death. When contacted, Director of Primary Health Care (PHC) Dr Mahbubur Rahman said, "We cannot rule out the possibility of the child dying in reaction to the vaccine (DTP). In fact, according to the World Health Organisation, death of one in three million from DTP vaccines is globally accepted and is very unfortunate for the child."
Dr Rahman further explained that all vaccines have 80 percent efficacy. The remaining 20 percent of the vaccines can cause accidents and it is globally accepted. Medical experts say a vaccine, like any medicine, is capable of causing serious problems, such as severe allergic reactions. But the risk of DTP vaccine causing serious harm, or death, is extremely small. Dr Rahman said that EPI officials are investigating the incident. While asked about the removal of the vials manufactured from the same batch, Dr Rahman said, "We can't order removal of the vials made in the same batch because it's troublesome and those vials have already been scattered across the country."
The EPI services in Paikgachha upazila remained suspended, health directorate sources said.
http://www.pennlive.com/news/patriotnews/index.ssf?/
base/news/1090402889100480.xml
New shots await 1,200 who got faulty vaccine
Wednesday, July 21, 2004BY DAVID WENNER
Of The Patriot-News
About 1,200 people vaccinated last year at Hamilton Health Center in Harrisburg need to get the shots again because the vaccine was stored in a faulty refrigerator and may have been ineffective. Those affected, mostly children, were inoculated at the Hamilton clinic at 1821 Fulton St. from February through October 2003. Officials said the vaccines in question pose no health threat.
The vaccines protect against debilitating childhood diseases such as diphtheria, tetanus and pertussis, hepatitis B, and measles, mumps and rubella. Jeannine E. Peterson, CEO of Capital Region Health System @ Hamilton Health Center, said the vaccines were stored in a refrigerator that sometimes chilled items to below freezing and below acceptable temperatures.
She said officials aren't certain whether the vaccines are ineffective. But she stressed the importance of the vaccinations and said Hamilton wants to make sure its clients have adequate protection. Hamilton has received no complaints from those who received the vaccine, she said. "We're not in a panic. This is not an outbreak. This is not something we feel [vaccine recipients] need to be overly concerned about," she said. Alice Gray, director of the Pennsylvania Department of Health's division of immunization, also said the vaccine itself poses no health threat. Revaccinations will be free. The health department provided vaccine for the revaccinations at no charge and offered to provide help in giving the shots, she said.
The health department discovered the problem during a routine inspection about three months ago. It used temperature records to determine the affected vaccine had been dispensed only from February through October, Peterson said. Peterson said it took several months for Hamilton to obtain advice from the department and from the U.S. Centers for Disease Control and Prevention on whether Hamilton should revaccinate people who received the vaccine, and to devise a plan.
Capital Region Health System @ Hamilton Health Center is a federally funded health clinic that serves mostly the poor. One of its missions is to reach out to people who might otherwise go without vaccines. Most of the people who received the potentially ineffective vaccine lived in Dauphin County. Some adults also received the vaccines. Peterson said many of the vaccine recipients move frequently, and it is unlikely that letters will reach all of them.
She said Hamilton is working with schools and day-care centers to find those who might need revaccinated. The letters, mailed yesterday, inform clients they need to return to Hamilton for re-vaccination. Staff will use Hamilton records to determine which vaccines the clients need. Hamilton will expand its hours to accommodate the re-vaccinations, Peterson said.
DAVID WENNER: 255-8172 or dwenner@patriot-news.com
http://www.thisislancashire.co.uk/lancashire/leigh/news/LEIGHNEWS4.html
Wrong son got MMR jab - mum
A YOUNG mum is to lodge an official complaint against her family doctor after a vaccination mix-up claim. Emma Hook, 23, and her partner Ian Whalley have started the procedure rolling after her life-long GP gave her 15-weeks-old son Ben Whalley the MMR vaccination meant for his two-years-old brother, Scott.
They claim the doctor should be struck off the medical register and have sent a letter of complaint to the Health Care Commission in Manchester. Emma, of Devonshire Road, Atherton, says she took her two sons to the surgery last Thursday for Scot to have his MMR jab and baby Ben, who was born prematurely, to have his first injections.
She alleges that after Ben was given his injection she was sent out of the surgery and called back later to be told that he had been given the wrong one. "I went numb, I didn't know what to do. Babies are not supposed to have the MMR jab until they are at least 13 months old. We were advised to take him to Wigan Infirmary and there staff rang the Department of Health to see what might happen. Ben was examined by specialists, who told us we were lucky he was under six months old as his immune system was not fully developed. "He had a very high temperature but thankfully he now seems OK. Had he been two months older what would have happened then?
"I am very angry, how can a doctor mix up two injections and give them to the wrong children? I want to make sure this does not happen to anyone else. "He has been my doctor for 23 years, and this is not the first trouble I have had, but it is the first and last time that Ben will see him. I will change doctors and the children will have their injections then." A spokesman for the surgery refused to comment. A spokesman for the Health Care Commission said information was confidential but the couple would have been advised that to follow the NHS complaints procedure they should first lodge a complaint with the practice manager and the complaints manager at the Primary Care Trust.
http://www.nytimes.com/aponline/national/AP-Tainted-Flu-Vaccine.html
The New York Times
August 27, 2004
A Big Maker of Flu Shots Finds Some Contaminated
By LAWRENCE K. ALTMAN
The Chiron Corporation, a major manufacturer of influenza vaccine, said yesterday that it had detected a contamination problem in its new supply and was delaying release of nearly 50 million doses until scientists could determine the cause. Chiron makes about half the 100-million-dose supply that federal health officials expected to be used this flu season. Its chief executive, Howard Pien, said in a telephone interview that it informed the Food and Drug Administration and the Centers for Disease Control and Prevention about the problem on Wednesday. Other than a relatively small amount sent to distributors in July, Mr. Pien said, the company will probably not begin issuing the vaccine until mid-October, about a month later than expected.
Chiron's disclosure to the government agencies came on the very day the Bush administration released the nation's first plan of preparation and response involving the next pandemic of influenza. In that plan, still in draft form, the government underscores a need to help industry improve the production of influenza vaccines for the ordinary seasonal outbreaks as well as for a pandemic. Dr. Julie L. Gerberding, the director of the disease control centers, said Chiron's contamination problem was "not good news," in part because the usual October flu immunization clinics may need to be postponed until November.
But given what Chiron has told her, Dr. Gerberding said in a phone interview, "at this point we don't anticipate an overall shortage.'' As a result of that expectation, she added, the C.D.C. is not now changing its recommendations as to who should be immunized. There are 185 million Americans who ought to be immunized under this year's recommendations, which, for the first time, advocate giving the vaccine - two doses, in fact - to all infants from 6 months to 23 months. In a usual flu vaccination season, most shots are given by Thanksgiving. But a number of public health experts expressed concern yesterday that the delay caused by the Chiron problem would complicate such efforts, because they require considerable planning. The experts said they worried that Chiron was offering a best case.
"It's a mess," said Dr. William Schaffner, chief of preventive medicine at Vanderbilt University. "This will lead to striking disparities in flu vaccine availability around Your Town, U.S.A.'' "If anything,'' Dr. Schaffner said, "this emphasizes the importance of the pandemic plan and the need for more vaccine manufacturers and new immunization techniques.'' Mr. Pien, Chiron's chief executive, said he did not foresee a widespread problem for his company's product, Fluvirin, because, he said, only 8 of 60 batches tested so far have failed to meet the manufacturer's sterility specifications.
"This should have a minimum impact on the American population," he said. Nevertheless, "no assurances can be given that additional tests on Fluvirin will yield satisfactory results or that Chiron will be able to release Fluvirin this season," the company said in a news release. Chiron (pronounced KY-rohn), based in Emeryville, Calif., outside Oakland, manufactures the vaccine in Liverpool, England. Its quality controls cover a wide range of tests, said Mr. Pien, who declined to specify which of those the eight contaminated Fluvirin batches had failed. He said the contamination was biological in nature and possibly due to human error, but again would not be more specific.
The four million doses in the eight batches will be destroyed, Mr. Pien said. He said that though Chiron shipped the first one million doses of Fluvirin to distributors in July, they had not been distributed to doctors and clinics. Mr. Pien said he thought the company would be able to release the rest of its full complement - from 46 million to 48 million doses - by mid-October. Though that would be about a month later than expected, he said, it would still be in time to meet public health needs for this influenza season. Chiron is manufacturing about 25 percent more vaccine than it produced last season. A second major manufacturer, Aventis, said it was ahead of schedule in producing more than 50 million doses. A spokesman for the company said it was surprised by Chiron's announcement and planned to determine whether Aventis could make more vaccine and, if so, how soon.
A third manufacturer, Medimmune, plans to manufacture from one million to two million doses of its FluMist, an influenza vaccine that is sprayed into the nose.
Denise Grady contributed reporting for this article.
Related Stories:
http://money.cnn.com/2004/08/27/technology/chiron.reut/
The Himalayan Times Online
Printed from www.thehimalayantimes.com
Kids critical after vaccination
Himalayan News Service
Biratnagar, September 27:
A medical team has been sent to Dhungesanghu of Taplejung district, where over three dozen children had taken ill three days after being administered anti-measles vaccine on September 21. The health team included WHO representative Jit Bahadur Tam-ang, one representative each from the Regional Education Directorate and District Public Health (DPH), said DPH, Taplejung. RSS said the condition of ten is reported to be critical. The children had vomited blood and have sores in their mouths, locals said. They were administered the vaccine during first phase of the the National Anti-Measles Vaccination Campaign. Asmita Subba, Puspa Subba, Mamata Maswa and Babita Maswa of Dhungesanghu-1, and Sushant Tamang and Januka Shrestha of Ward Nos 4 and 5 of the same VDC are reported to be critical. The sick children's parents said their children suffered from cough, chest pain, high fever, loss of appetite and vomitted blood after being injected with the anti-measles vaccine. Jagannath Adhikari, headmaster of the Party Primary School in Fatyangre, said 25 students have not come to the school after getting the vaccine.
COPYRIGHT@ 2004 THE HIMALAYAN TIMES PUBLICATION. All rights
reserverd
Claims that kids ill after vaccination ‘false’ Himalayan News Service
Kathmandu, September 28:
A government official said today that the reports of children taken ill after the anti-measles vaccination are false. “The medical team which visited Dhungesanghu in Taplejung has submitted its report saying that the children were only suffering from pharyngitis and common cold,” said Bal Krishna Suvedi, of the immunisation department of Child Health Division. “They claim that the illnesses has nothing to do with the vaccination.” The Eastern Region Health Directorate, that had sent a team to the VDC has issued a press release today saying that none of the children were in serious condition. “Some of the children had been suffering from cough, headache and nausea and upon examination, were found to have pharyngitis and cold. The children are better now.” Suvedi also said that the reports in the media which claim children coughing up and vomiting blood are ‘totally wrong’. No other cases of such complications have been reported yet said the official.
The national anti-measles campaign, which is to be implemented in three phases, targets to vaccinate 9.5 million children, between the ages of nine months to 15 years throughout the country. The first phase, which started on September 21, vaccinated children in the eastern and central region. In the second phase, beginning on January 4, 2005, the children in 34 districts of western, mid-western and far-western regions will be vaccinated. The third phase of vaccination, starting on April 16, 2005, will target the six himalayan districts. Around 1,50,000 children across the country suffer from measles every year. About 2,669 measles-related death was reported in the year 2003. So this anti-measles campaign aims to reduce the deaths by half and bring it down to 1,335 by the year 2005.
http://timesofindia.indiatimes.com/articleshow/874894.cms
Polio virus escaped twice from laboratories
TIMES NEWS NETWORK[ WEDNESDAY, OCTOBER 06, 2004 01:25:10 AM ]
NEW DELHI: The polio eradication exercise is beginning to acquire shades of a science thriller. Not only has the virus escaped twice from laboratories in the country, but also caused the crippling disease, acute flaccid paralysis in 10 persons.
The government has now started a mammoth exercise to gather a list of laboratories storing the polio virus before it can claim to have eradicated it. Contrary to popular knowledge that the polio virus is transmitted only through contaminated food and water, it can also be passed on by an infected person through respiratory secretions, such as coughing and sneezing. Laboratory strains of the virus were identified earlier in three polio-stricken cases in 2000 and another seven more recently.
The government is well aware that the entire eradication effort could be at risk if the virus were to escape from different laboratories. A task force under the chairmanship of the director-general of the Indian Council of Medical Research (ICMR), N K Ganguly, has been set up, which is expected to come up with a detailed list of laboratories storing the virus. But this may well take a good two years. ICMR has already written letters to all ministries to give a list of laboratories under them.
Experts explain that this is a formidable task: India has a large number of private laboratories. One, enumerating these laboratories may not be easy as there is no legal or other requirement for their registration and second, even collection of reports from them would be quite formidable. A district list would need to be prepared with help from the district health officers.
Vet Microbiol. 2004 Sep 8;102(3-4):131-40. Related Articles, Links
Comparison of the sensitivity of in vitro and in vivo tests for detection of
the presence of a bovine viral diarrhoea virus type 1 strain.
Antonis AF, Bouma A, Bree Jd J, De Jong MC.
Division of Infectious Diseases and Food Chain Quality, Animal Sciences
Group, Wageningen University and Research Centre (WUR), P.O. Box 65, Lelystad
AB8200, The Netherlands.
Veterinary vaccines are usually tested for the absence of contaminants. However, the quality control does not always imply that vaccines are not contaminated as, for example, illustrated by the bovine herpes virus 1 (BHV1) vaccine used in The Netherlands in 1999 that contained a small amount of bovine viral diarrhoea virus (BVDV1). Thousands of cows were vaccinated with BHV1 vaccine batches, and the question arose as to whether these small amounts of BVDV1, most likely not detected with in vitro tests, could have infected cattle. More in general, the question was whether the outcome of the in vitro tests, i.e. the in vitro infectivity, was indicative for the infectivity for cattle, i.e. the in vivo infectivity. We therefore carried out in vitro experiments to determine the sensitivity of a BVDV1 isolation assay. In addition, we performed two animal experiments, in which we estimated the lowest dose needed to infect calves with BVDV1. We extrapolated the experimental in vitro and in vivo results from a tissue culture infectious dose (TCID(50)) to a cattle infectious dose (CID(50)). We observed a partial response in the calves inoculated with this dose: four out of six calves turned out to be infected. In the tissue culture test, all 20 samples tested negative. The response in vivo, however, was not significantly higher than the in vitro response, which implies that no difference in susceptibility was observed between the animal test and the tissue culture test. Based on the results in our experiments, some cattle may have been infected with BVDV1 after the application of the contaminated BHV1 vaccine during the vaccination campaign. The question remains that how many cattle received contaminated vaccine, and became infected with BVDV1.
PMID: 15327789 [PubMed - in process]
http://www.nepalnews.com.np/archive/2004/sep/arc_sep04_43.htm#3
Three dozen children fall sick following immunization against measles
Three dozen children in the Dhungesanghu VDC of Taplejung district, who were vaccinated against measles in the recently held immunization campaign, have fallen sick. The state of ten of them is reported to be critical. Some of the sick children have developed cough, fever, chest pain, shivering, loss of appetite and wounds in the mouth while some have even vomited blood, Nepal Samacharpatra reported today, quoting parents of the sick children. The children were vaccinated against the deadly disease during the firstphase of the immunization campaign held on September 21. “I don’t know what happened. But my daughter has become ill after she was administered the vaccine,” the daily quoted one Ambika Nepali, a local guardian, as saying.
Following the news, the Taplejung district hospital had dispatched a medical team to the VDC, which lies some 12 kilometers away from the district headquarters. Though the team distributed antipyretic drugs like Cetamol and Ketomox in the village, more children fell sick, the daily said quoting another local named Durga Maskey.
nepalnews.com amt Sept 27 04
http://kyw.com/Local%20News/local_story_302163549.html
Oct 28, 2004 6:08 pm US/Eastern
PHILADELPHIA (KYW) A lab worker from Jefferson Hospital has been contaminated with a virus that is used in the smallpox vaccine. Medical Reporter Stephanie Stahl has learned that the female worker was treated at Wills Eye Hospital for an eye lesion. She was then sent back to Jefferson Hospital where she remains under quarantine. The Philadelphia Health Department confirms that the incident is a case of Vaccinia and it has in fact been isolated.
Stahl reports that Vaccinia is the virus that’s used in the creation of the smallpox vaccine. The virus is related to smallpox but is a much milder and less deadly form that is frequently used for research purposes. In addition, the virus is sometimes used in genetic research to help create other vaccines. Vaccinia can in no way cause smallpox and Stahl says that there is no public health concern as the situation is contained. However, the virus is contagious with close contact. Officials from Jefferson and Wills Eye Hospitals refuse to comment about the situation, citing it is a violation of patient confidentiality. At this point, the condition of the worker is unclear.
http://www.mlive.com/news/fljournal/index.ssf?/base/news-24/1099932606257390.xml
Woman says overvaccination caused son's autism
FLINT
THE FLINT JOURNAL FIRST EDITION
Monday, November 08, 2004
By Shantell M. Kirkendoll
skirkendoll@flintjournal.com • 810.766.6366
QUICK TAKE
Too much of a good thing?
* Children can end up with too many vaccinations or be denied entry to school if state records are not updated. Parents should be sure their doctor or clinic reports vaccines to the Michigan Childhood Vaccination Registry within seven days of a child's appointment, and keep a paper record of vaccinations as backup.
FLINT - Syrus Davis' mother wanted him protected from measles, mumps, hepatitis and polio, but at age 6 he's had nearly twice as many immunizations needed to protect him from those diseases. It's been too much of a good thing, said Yashica Neal of Flint. She said she believes the childhood vaccines poisoned Syrus, who was recently diagnosed with autism.
She blames her pediatrician's office for failing to report vaccinations on time to a state registry designed to prevent overvaccination. Because of the snafu, Syrus received more shots than he needed, she said. "He changed after all those shots," Neal said. "He's gloomy and lost." While most children need only four doses of the polio vaccine, Syrus has had six doses, according to state records. By 1999, he had five doses of Hepatitis B vaccine, when he only needed two. It's been only recently that Syrus started talking. Late speech is a classic sign of autism in which children are socially withdrawn and lack language skills.
Like many parents and autism groups, Neal contends the mercury in vaccines is linked with autism. That contention is hard to dismiss because of a marked increase in autism in the past decade that experts cannot explain. Research has been little help. The Institute of Medicine issued a report in May concluding no causal link between vaccines and autism, but a month later, a study published online by the journal Molecular Psychiatry showed it did cause autism-like symptoms in some newborn mice.
But "no one has studied what happens when children are overvaccinated," said Neal, 29. "It couldn't have been good for him." Syrus was born in 1997, the same year the Michigan Childhood Immunization Registry went into effect. All health care providers who give vaccines are required by law to report every vaccine they give a child.
It's supposed to help parents keep track of vaccines administered at multiple places such as a clinic or doctors' offices. MCIR is considered more reliable than paper records, which can be lost or destroyed. "But when you look up in the system, the shots from the pediatrician are not there," she said. "I think that's what's been going on." Neal took care of Syrus' shots with a pediatrician her family had used for years, but she claims his office reported shots weeks after appointments or not at all.
After Syrus was born, Genesys Home Health and Hospice's Maternal Infant Support Service came to Neal's home to check on his well-being and hers and, based on the MCIR records, gave Syrus vaccinations. A Genesys spokeswoman said Friday that nurses use MCIR as the "gatekeeper" for children's records, and follows federal guidelines for when children need vaccines.
"I remember the day (the nurse) came to the house," Neal said. "He'd already had the shot last month. I knew that, but she said the state record showed he hadn't. Who was I to deny medical help for my son? I listened to her." Most children will have five doses of vaccine to prevent diphtheria, tetanus and pertussis, but Syrus has had seven doses, according to his MCIR record.
Parents may also feel strong-armed into getting vaccines because children cannot attend school or preschool unless records show they have all their shots. Neal became alarmed about the shots when Syrus' preschool told her in June he wouldn't be able to attend school because state records showed he needed another measles-mumps-rubella shot. Syrus' doctor's records show he had the
shot in October 2001.
Syrus' pediatrician did not return calls seeking comment. Neal has contacted an attorney who may file a lawsuit against him, she said. "Making sure the data is there is something providers have to buy into," said Wendy Nye, director of the local MCIR region. "Most recognize it as a wonderful tool for tracking vaccinations." Compliance may be affected by an office's volume of vaccinations to be entered or turnover of staff trained to do it, she said.
She said 95 percent of Genesee County doctors are connected to the registry and vaccinations must be on the registry within seven days. "Occasionally parents dispute MCIR, and that's usually when a child has more shots on their (immunization card) from the doctor's office than the registry," she said.
Generally, schools will accept a doctor's note or record as proof of a vaccination, even if MCIR shows otherwise, Nye said. Health officials say few vaccines given U.S. children today contain the preservative thimerosal, which is used to store multiple doses of some vaccines such as for hepatitis B. Thimerosal contains mercury.
The U.S. Centers for Disease Control and Prevention and the American Academy of Pediatrics in 1999 recommended a switch to mercury-free vaccines. Most of Syrus' shots were given more than two years before the switch. "The amount of shots Syrus has gotten is unacceptable," said LaQuantus Cardwell, 30, his aunt and an office manager in a pediatrician's office. "No one knows whether it was intentional, but we do know Syrus will be this way for the rest of his life."
© 2004 Flint Journal.
http://www.sun-sentinel.com/news/local/florida/sfl-1020flushots,0,3196621.story
?coll=sfla-news-florida
"In Central Florida, the vaccine mix-up was discovered Monday, when Get Healthy Florida received about 6,000 doses of influenza vaccines labeled "Shire." "Shire Pharmaceuticals Group is a British company that sold its vaccine division in September to a Canadian company, ID Biomedical."
http://news.bbc.co.uk/1/hi/wales/mid/4017559.stm
Vaccine scare boy 'improving'
A teenager is "improving" in intensive care after a severe allergic reaction to a vaccination at a school in Aberystwyth. Dominic Hamer, 13, is off a ventilator and talking. It is hoped he will be transferred to a paediatric ward at Bronglais Hospital later.
Ten other pupils from Ysgol Gyfun Penweddig were also treated after "adverse effects" to the injections. Two other pupils will be discharged on Wednesday, said the hospital. Nearly 120 pupils at the comprehensive school were being injected with the BCG vaccine, which combats tuberculosis.
A Ceredigion NHS Trust spokesman said it was investigating, and had stopped the batch of vaccine being used. Dominic was said to have had an anaphylactic shock, which is defined as a very sudden serious physical reaction caused by an allergy. Three fellow pupils were kept in overnight for observation, but the rest were discharged.
Bronglais Hospital chief executive Alison Williams said Dominic had improved overnight. She said on Wednesday that medical staff "hope to move him to a paediatric ward later today and send him home at the end of the week". The trust has said it is working closely with the school to ensure that
pupils and parents receive any information or support they require. Individual advisory letters have also been issued to pupils. Brian Thomas, head of corporate services at the trust, confirmed the batch of vaccine had been withdrawn.
Intensive care
A statement from the trust said the batch of vaccines used at the school had also been used on Monday without any adverse effects. A team of four doctors and three nurses had been conducting the vaccinations at Penweddig Comprehensive.
The statement said: "At 12.30pm, nine children presented at the accident and emergency department of Bronglais General Hospital following a suspected adverse reaction to a BCG immunisation. "Another two pupils attended the department in mid afternoon." "Following assessment, 10 of the pupils were admitted for observations and one pupil admitted to the intensive care unit."
Reaction
Dr Mike Simmonds, the Welsh Assembly Government's Senior Medical Officer, said youngsters who are receiving the BCG vaccination are first given a "star" injection in their forearm. "If you don't get a reaction to that you then go on to get the actual vaccine which is given actually within the layers of the skin".
Dr Simmonds told Radio Wales that reactions to vaccinations to children were "incredibly rare". Only 80 cases had been identified in a three-year period among the millions of vaccinations administered across the UK. "All these diseases that we are protecting against have had a horrible track record," he said. "TB is a long-term illness. In the past people were in hospital for weeks and weeks if they survived that illness. "Measles just kills and can kill very rapidly, and is still one of the biggest killers across the world".
http://www.newindpress.com/Newsitems.asp?ID=IEA20041122141524&
Title=Southern+News+-+Andhra+Pradesh&Topic=0
Infants die; reaction to polio drops feared
Tuesday November 23 2004 00:36 IST
KHAMMAM: In a shocking incident, three infants died, after allegedly developing reaction to polio drops administered on Sunday in Penuballi mandal of this district.
According to information reaching here, all the three infants, aged under two months, suffered from shivering after being given polio drops. One kid died on Sunday and two others succumbed on Monday. Unconfirmed reports said that another infant died in the mandal due to a similar reaction.
The victims were identified as R Venkat Narsaiah of Bramhalakunta, Pramila (Karaigudem) and another baby girl who is daughter of one K Narasimham Kumar in Upplachalaka village. The parents alleged that the deaths were a result of complications arising out of polio drops and that the children were normal and healthy before.
Dr Gopala Krishna and Dr Ranga Prasad from Hyderabad, who are a part of the rapid response team of WHO, visited the district to obtain a report on the deaths.
When contacted, district medical and health officer Dr R Krisha Bramham maintained that the deaths were unrelated to polio drops. ``It is a coincidence that the deaths happened just a day after polio drops were given,'' he said.
Polio dropped from list of suspects
http://timesofindia.indiatimes.com/articleshow/935121.cms
November 25, 2004
HYDERABAD: Two more babies who had been given polio drops last week died at Penuballi and Aswaraopet mandals in Khammam district on Tuesday, but experts and government officials took pains to explain that the vaccine could not possibly have caused the deaths. These two deaths and one more in Anantapur on Tuesday took the number of post-inoculation mortalities to seven.
A postmortem on the exhumed bodies of four children who had died over the weekend showed that they had succumbed to neonatal complications. Health officials said none of the seven deaths could be attributed to the oral polio vaccine drops. Nevertheless, the incident is being "critically viewed" and samples of the vaccine given to the children are being sent to the Central Drug Research Institute at Kasauli.
Family welfare officials said the children, most of them months old, either had a medical history or died of reasons not linked to the polio drops. It was a coincidence that the deaths have occurred after inoculation.
Dr R Gopalakrishna Rao, joint director of the family welfare department, said two of the Khammam deaths were due to asphyxia (suffocation) caused by vomit— "not caused by the polio drops"—and one was due to intestinal obstruction. Some of the deaths could have occurred due to some substances being administered to the babies by the mothers: one had received oil drops in her nose and one was given a sugar syrup.
One of the seven children who died was a premature baby who had developed respiratory problems after swallowing amniotic fluid during birthr. A five-year-old girl who died in Kurnool was suffering from fits, Dr Rao said.
"The deaths are a sheer coincidence. We are 100 per cent sure that the deaths have nothing to do with polio drops," Dr Rao said.
Principal secretary (medical and health) I V Subba Rao said the post mortem reports proved that the deaths were due to the vaccine.
http://elmundosalud.elmundo.es>
The Sanitation Ministry is retaining a batch of yellow fever vaccine
--------------------------------------------------------------------
The Sanitation Ministry reported today [26 Oct 2004] that they are retaining a batch of yellow fever vaccine as a "preventive measure," after a 26-year-old woman from Onuba died from yellow fever stemming from a post-vaccination reaction. The woman was admitted last Thursday [21 Oct 2004] to Juan Ramon Jimenez Hospital in Huelva because of fever and multi-organ failure, having had, in addition to fever, malaise, vomiting, and diarrhea during the previous days.
According to a press release from the hospital, since she was about to travel abroad, the patient was also vaccinated for diphtheria and tetanus on 14 Oct 2004 at the External Sanitation Services in Huelva, an office of the Sanitary and Consumer Affairs Ministry. The patient was directly admitted to intensive care, where she was diagnosed with yellow fever associated with a post-vaccine reaction that rapidly progressed and could not be successfully controlled by the medical team, and she died last Friday [22 Oct 2004] at 5:30 AM.
Sources from the Sanitation Ministry reported that they are waiting for new information from the Andalucia Council regarding this case. They also emphasized that people "should still have their vaccines if they have to travel abroad," since the benefits of immunization outweigh the risks.
No past history of such an event in Spain
-----------------------------------------
The same sources gave assurances that the performance of the Huelva Vaccination Center was "perfect" with respect to the protocol, and they have tried to avoid generating unnecessary alarm, insisting that "the batch has been localized."
The Sanitation Ministry commented that "since 1985, there have been only 25 communications reporting mild symptoms considered as vaccine reactions, but not a single case similar to that reported in Huelva." Accordingly, the Sanitation Ministry indicated that the frequency of cases similar to the one reported today [26 Oct 2004] for the age group between 25 and 44 years is less than zero percent per 100 000 inhabitants. The Ministry is waiting for information about the case from the
Andalucia Council; consequently, the vaccine batch retention "is only a preventive measure issued by the Sanitation Ministry," they pointed out.
The Management at Juan Ramon Jimenez Hospital confirmed that samples were sent to the Majahonda National Center for Microbiology and Virology soon after the victim was admitted to the hospital for biologic confirmation of the case.
Yellow fever vaccine, which is manufactured using attenuated viruses, is not included in the vaccine calendar of the Andalucia Council, since it is a disease absolutely absent in Andalucia and in Europe. The batch immobilization is a measure recommended by the External Sanitation Department, which belongs to the Ministry of Sanitation and Consumer Affairs, to be complied with only in case of traveling to countries where the disease is still present: i.e., the majority of sub-Saharan countries and tropical countries in the Americas.
--
Pablo Nart
<p.nart@ntlworld.com>
[Brazil, USA, and Australia have also reported such cases (see refs. below). This case does not seem to correspond to problems with the vaccine (contaminated batches), or with manufacturing problems, but is a consequence of a special interaction between the virus in the vaccine and the host; since, in rare cases, there might be an inability by the host to generate an adequate immune response to the virus. - Mod.LJS]
[see also:
2002
----
Yellow fever vaccine-associated deaths - MMWR report 20021108.5756
2001
----
Yellow fever vaccine-associated deaths reported 20010715.1367
Yellow fever vaccine-associated deaths (02) 20010727.1467
Yellow fever vaccine-associated deaths (05) 20011201.2925
2000
----
Yellow fever - Brazil: vaccine deaths 20000326.0436
Yellow fever - Brazil: vaccine deaths (02) 20000328.0452]
http://www.sunnetwork.org/news/regional/andhra/andhra.asp?id=10860
Sun Network, India
Four babies `die after polio vaccination'
Khammam, Nov 23 - Four babies, all hardly two-months-old, died in Penuballi and Sathupalli mandals within 24 hours of the pulse polio immunisation programme. Parents of the babies blamed it on the side effects of the vaccine. Members of the rapid response team deputed by the World Health Organisation and medical teams from Hyderabad were on their way to the villages.
According to reports, Laksmi, a two-month-old baby, died in Uppalachalaka village today. She was administered the pulse polio vaccine around 9.30 a.m. A two-month-old baby, Rajaneni Venkatanarsaiah, died in Brahmalakunta village in the day, after being administered the pulse polio drops around 10 a.m on Sunday.
Some 145 children below two years were covered under the pulse polio vaccination programme in the village. The medical and health authorities said that all others who were given the vaccine were safe.
http://timesofindia.indiatimes.com/articleshow/935121.cms
The Times of India
Polio dropped from list of suspects
THURSDAY, NOVEMBER 25, 2004 12:43:44 AM ]
HYDERABAD: Two more babies who had been given polio drops last week died at Penuballi and Aswaraopet mandals in Khammam district on Tuesday, but experts and government officials took pains to explain that the vaccine could not possibly have caused the deaths. These two deaths and one more in Anantapur on Tuesday took the number of post-inoculation mortalities to seven.
A postmortem on the exhumed bodies of four children who had died over the weekend showed that they had succumbed to neonatal complications. Health officials said none of the seven deaths could be attributed to the oral polio vaccine drops. Nevertheless, the incident is being "critically viewed" and samples of the vaccine given to the children are being sent to the Central Drug Research Institute at Kasauli.
Family welfare officials said the children, most of them months old, either had a medical history or died of reasons not linked to the polio drops. It was a coincidence that the deaths have occurred after inoculation.
Dr R Gopalakrishna Rao, joint director of the family welfare department, said two of the Khammam deaths were due to asphyxia (suffocation) caused by vomit- "not caused by the polio drops"-and one was due to intestinal obstruction. Some of the deaths could have occurred due to some substances being administered to the babies by the mothers: one had received oil drops in her nose and one was given a sugar syrup.
One of the seven children who died was a premature baby who had developed respiratory problems after swallowing amniotic fluid during birth. A five-year-old girl who died in Kurnool was suffering from fits, Dr Rao said. "The deaths are a sheer coincidence. We are 100 per cent sure that the deaths have nothing to do with polio drops," Dr Rao said. Principal secretary (medical and health) I V Subba Rao said the post mortem reports proved that the deaths were due to the vaccine.
http://abcnews.go.com/Health/wireStory?id=232833
Doctor Accused of Using Old Flu Vaccine
Washington State Accuses Physician of Giving Outdated Flu Vaccine to 55 Patients
BELLINGHAM, Wash. Nov 6, 2004 — The state's medical quality board has accused a physician of giving outdated flu vaccine to 55 patients. Dr. Gary McCallum used a batch of last year's vaccine, according to the state's Medical Quality Assurance Commission, which licenses physicians and other medical professionals. The board charged him on Friday with unprofessional conduct, specifically fraud and misrepresentation. McCallum told patients the vaccine, purchased last year in Canada, was good for the current flu season even though its expiration date was in July, state Health Department spokesman Tim Church said. "The people that received the vaccine aren't at any increased risk of side effects, but they may think they've been immunized when they … have not been immunized," said Regina Delahunt, director of the Whatcom County Health Department.
Flu vaccine is custom-made each year for the types of virus expected during the coming flu season. About half of the nation's supply for this winter was eliminated because of contamination at one drug company's laboratories in Britain.
McCallum, of Barkley Village Family Medical Clinic, said all affected patients were getting new shots and that he could not comment further. McCallum administered the vaccine between Oct. 15 and Tuesday, charging $20 for adults and $10 for children. Health Department investigators visited McCallum's practice Wednesday after receiving a tip, Church said.
http://www.newsday.com/news/nationworld/wire/sns-ap-injection-scare,0,970987,print.story?coll=sns-ap-nationworld-headlines
Nurse in Flu-Shot Scare Claims Innocence By JOSHUA FREED
Associated Press Writer
December 13, 2004, 5:07 AM EST
MINNEAPOLIS -- The woman offering flu shots for $20 in the commons area at Augsburg college seemed plausible enough -- green scrubs, white lab coat, stethoscope -- that some three dozen people willingly paid their money, rolled up their sleeves and let her plunge the needle in.
But no one had scheduled a flu clinic. No one knew who the woman was. And no one could be sure what was in those syringes. She turned out to be 33-year-old Michelle Torgerson, a freelancing nurse who claims she was selling leftover vaccine to raise money for a fund-raiser at her daughter's school. And those shots were certainly nothing sinister, if not just plain old flu shots, lab tests have found.
But in an age when bioterrorism experts worry about sophisticated attacks, the case shows how anyone with a syringe and a reasonable looking get-up can have potential victims lining up -- even paying -- to be injected with who-knows-what.
"Imagine if they hadn't been able to track her down, and they had no idea, and somebody had just come onto a college campus injecting people with something," said Dr. Michael Osterholm, a bioterrorism expert with the National Center for Food Protection and Defense at the University of Minnesota.
Osterholm said it's not realistic to expect patients to challenge the credentials of every nurse who administers a flu shot -- but he said the school should have. "I think this really is at the center of the discussion about what does it mean to recognize and report suspicious behavior," he said. On Dec. 2, a staffer confronted Torgerson, asking who she was and who her supervisor was, according to Augsburg security director John Pack. Pack said he and another security worker headed toward the commons to investigate. "At that point I wasn't thinking whether or not it was malicious or a mixup," Pack said. "I was thinking about the scope -- I wondered how many people received shots."
Torgerson, of suburban Albertville, had left the campus promising the staffer she would provide the name of a supervisor, Pack said. But the security director said she never did, further raising suspicions.
Torgerson gave a different account Sunday, saying she did speak briefly to an administrator named "Diane," but that she wasn't challenged. She said she left after the noon hour, as she had planned, and did not "abruptly" leave, as officials have said. Pack called police, and then set about trying to find out how many people had been injected. He sent an e-mail alert to everyone on campus, and printed red fliers asking anyone who had gotten the shots to come forward. Teachers read the alert in class the next day.
Police arrested Torgerson the day after the injections were discovered, near her ex-husband's home in Belgrade, 90 miles away from the college. Torgerson, a licensed practical nurse who had given immunizations as part of a legitimate clinic at Augsburg last month, insisted Sunday she did nothing wrong. She said she believed she did have permission from an Augsburg administrator in charge of the student center to give the flu shots on her most recent visits to the school.
Torgerson has not been charged.
But some students are going in for HIV tests anyway, and about 100 attended a meeting in the school's chapel last week where police and health workers answered questions. Augsburg president William Frame apologized. Some students were mollified, while others remained angry. According to the Minnesota Department of Health, test results that came back Friday from the Food and Drug Administration showed the vials police took from Torgerson contained real flu vaccine, but the vaccine in some vials had been watered down with saline solution. On Sunday, Torgerson emphatically denied diluting the vaccine, and her lawyer disputed the accuracy of the test results, saying they'll bring in their own experts.
Torgerson, who had worked at several immunization clinics, has said her employer told her to dispose of the leftover vaccine. Employer Maxim Healthcare Services of Columbia, Md., said Torgerson was told to return all her unused vaccine by Nov. 12, the day after her last authorized flu clinic. Torgerson disputed that too, saying her supervisor told her was trying to line up other flu shot clinics.
Some bioterrorism experts said it's tough to guard against a theoretical threat like someone impersonating a nurse and injecting victims with something toxic. "Society operates at a certain level of trust, and I think it's very difficult to really police ... any sort of activity like this very extensively," said Dr. D.A. Henderson, a senior adviser at the Center for Biosecurity at University of Pittsburgh Medical Center.
"There are reasonable fears that we have, and there are a million potential things that we could imagine someone might do if they really were nasty about it," he said. Augsburg now requires outside vendors to display a permit. But Pack said Torgerson seemed to be trying to answer the staff person's questions, even promising to call back with the name of a supervisor.
"She certainly acted like she belonged," he said.
Associated Press writer Steve Karnowski contributed to this story.
Copyright © 2004, The Associated Press
http://www.woai.com/news/local/story.aspx?content_id=CFCBDB02-665F-4844-BEB1-73A84C1C0F51
Vaccination Double Dose
A local mom made sure her daughter was vaccinated against deadly diseases, but the state of Texas has now forced her to double dose her child. Now she's scared these repeat shots may be dangerous.
“Every time she comes down... getting sick, I'm going to worry,” Deborah Keller says of her daughter. “If she's slow at learning something at school, I'm going to worry.” Deborah's worried because her 4-year-old daughter was forced to repeat her polio, diphtheria and tetanus shots because of a new state law. The law went into effect after school nurses said they wanted immunization schedules to be based on grade level, not birthdates.
Her daughter's birth date is June 22nd. She received her shots June 11th. That brought a phone call from the school. “They called me last week - over a year after she's had her shots - telling me that in April of this year, the law has changed and she has to have those shots within four days of her birthday,” Deborah explained. “There are some that are getting caught in the crossfire.”
Deborah's daughter is one of them. She's in kindergarten
“The state has allowed first through 12th graders to be grandfathered,” she said. She says she never heard about the new law. “We had done some interviews for news and done some promotions in April and May,” countered Linda Lopez, a registered nurse at the San Antonio Health District. But no official notification.
“I don't know if the State made any announcement except on their web page,” said Lopez. But now it's the law; whether it makes sense or not. “The shots are good for ten years, whether she gets them eleven days before her birthday or four days before her birthday.” Health officials say the second round of vaccinations should not be harmful to Deborah's daughter. I
January 24, 2005 Exposure at Germ Lab Reignites a Public Health Debate By SCOTT SHANE
ast year, while working on a vaccine to protect against bioterrorist attacks, three laboratory workers at Boston University were exposed to the bacteria that cause a rare disease called tularemia, or rabbit fever.
The workers recovered, though two of them had to be hospitalized. But the prognosis is less certain for the university's ambitious plan to build a high-security biodefense laboratory, part of a national boom in germ defense research touched off by the Sept. 11 attacks and the anthrax letters of 2001.
The tularemia episode, acknowledged by university officials only after inquiries last week from the news media, has outraged opponents of the proposed $178 million laboratory and reignited a national debate over whether the rapid expansion in work with dangerous pathogens is adequately regulated and scientifically justified.
The Boston case follows other mishaps in germ research, including the accidental shipment of virulent live anthrax from Maryland to California last March, and an investigation that revealed multiple spills of anthrax bacteria in the Army's biodefense laboratory. Such incidents have led some scientists to ask whether the growing number of germ laboratories - financed from the $14.5 billion in federal money spent on civilian biodefense since 2001 - may pose a menace to public health comparable to the still uncertain threat from bioterrorism.
Dr. David Ozonoff, a professor of environmental health at the Boston University School of Public Health who originally supported the new laboratory but now opposes it, argues that biodefense spending has shifted money away from "bread-and-butter public health concerns." Given the diversion of resources and the potential for germs to leak or be diverted, he said, "I believe the lab will make us less safe."
Dr. Mark S. Klempner, associate provost for research at Boston University's medical school, says the proposed laboratory, to be designated a National Biocontainment Laboratory along with one to be built in Galveston, Tex., will pose no public hazard. To be designated Biosafety Level 4 - the highest level of security - it will develop drugs and vaccines to protect not only against bioterror agents but also such natural emerging diseases as SARS and West Nile virus, he said.
"The nation needs this lab," Dr. Klempner said.
Such disparate views among scientists reflect deep uncertainty about the scale and imminence of the bioterror threat. Some experts believe an attack that could kill tens of thousands of people is plausible today. Others argue that the known terrorist groups have little sophistication about biological weapons. Instead, these critics say, the biodefense expansion has been fueled by a scramble for federal money.
Currently there are four Biosafety Level 4 laboratories nationwide, with six more planned; 50 laboratories operate at Biosafety Level 3, sufficient to work with anthrax, and 19 more are planned at universities and government institutions, according to the Sunshine Project, a Texas group that is tracking the growth.
In the only major bioterrorist attack in American history - the anthrax-laced letters mailed to news media figures and two senators in fall 2001, killing five people - F.B.I. investigators have focused chiefly on the theory that the anthrax originated not with outside terrorists but within an American biodefense program.
By the same token, the critics say, the tularemia that sickened the workers in Boston would not have existed if not for bioterror research. Dr. Richard H. Ebright, a molecular biologist at Rutgers University, said the disease "has zero public-health importance." Only about 130 cases a year are reported in the United States.
The flood of biodefense financing has drawn hundreds of inexperienced researchers into work with hazardous organisms, Dr. Ebright said. The Boston accident, he added, "shows gross, basic incompetence and raises real questions about the competence of that institution to run a biosafety Level 4 lab."
Boston University officials concede that the tularemia vaccine researchers did not follow proper safety procedures and have removed the principal investigator, Dr. Peter A. Rice, from his post as chief of infectious diseases. Dr. Rice was to have had a role training workers for the proposed high-security laboratory.
University officials say the tularemia vaccine researchers thought they were working with a harmless "vaccine strain" of the Francisella tularensis bacterium. But for reasons unexplained, the sample was mixed with a virulent strain.
When two laboratory workers developed flulike symptoms in May, no one tied the illnesses to the research, said Dr. Thomas J. Moore, acting provost of the university's medical campus. Only after a third worker was hospitalized for several days in September did Dr. Rice first pursue the possibility of tularemia.
Dr. Moore said the tularemia diagnosis was confirmed on Oct. 29 but not reported to state health authorities until Nov. 9, a delay he said he could not explain. But he defended the decision not to tell the public.
"I feel comfortable about the decision not to make a public announcement because there wasn't a public risk, since tularemia can't be passed from person to person," Dr. Moore said.
Opponents of the proposed laboratory see things differently. Douglas H. Wilkins, a Boston lawyer who filed a lawsuit on Jan. 12 challenging the laboratory plan on behalf of 10 neighborhood residents, noted that the university's environmental impact statement claims the medical center has "not had any laboratory-acquired infections."
Tomas Aguilar of Alternatives for Community and Environment, a group opposed to the laboratory, said: "Three infections in a five-month period - and this is all going on when Boston University is saying how safe this dangerous laboratory is going to be. A lot of people are saying, 'It's even worse than we thought.' "
Similar safety questions have been raised by two other incidents. In 2002, the discovery of lethal anthrax outside a high-security laboratory at the military's premier biodefense laboratory, the Army Medical Research Institute of Infectious Diseases at Fort Detrick in Maryland, led to sampling throughout the institute. Investigators found three different strains of anthrax bacteria outside the sealed-off laboratories, indicating at least that many leaks, according to an Army report.
Then, last spring, Southern Research Institute, a contractor in Frederick, Md., shipped anthrax bacteria to an Oakland, Calif., hospital after immersing it in hot water to kill the germs. When mice injected with the supposedly harmless bacteria for a vaccine experiment quickly died, researchers realized the bacteria were still lethal.
Those incidents produced no human illnesses. But Dr. Ebright said some current research poses a much higher risk, notably the work by several groups that are trying to reconstruct the 1918 influenza virus, which killed more than 20 million people.
"This work is being done in the absence of any real oversight," he said.
One scientist who supports the increase in biodefense spending, Dr. Tara O'Toole, does not dismiss the safety issues. In fact, she said, the biodefense expansion has focused attention on long-neglected biosafety rules. But she believes the danger of bioterrorism is so great that the billions of dollars being spent on protections may not be enough.
"I think bioterrorism is the biggest national security threat of the 21st century," said Dr. O'Toole, director of the Center for Biosecurity of the University of Pittsburgh Medical Center. "So I want a robust biodefense research and development program."
Dr. O'Toole recently helped organize a bioterror exercise, called Atlantic Storm, in which terrorists attack with smallpox in the United States and four foreign countries, killing more than 87,000 people. Such a potential toll puts the risk of laboratory accidents in a different perspective.
But is that situation realistic, when nothing remotely approaching such an attack has ever occurred?
"After 9/11, I don't think anyone would say that just because an attack hasn't happened, it can't happen," Dr. O'Toole said. "I keep trying to talk myself out of this. But it just keeps getting scarier and scarier."
Parents still suspect BCG vaccine after many children fell ill at school Jan 26 2005
http://icwales.icnetwork.co.uk/0100news/0200wales/tm_objectid=
15116700%26method=full%26siteid=50082%26headline=%2dparents
%2dstill%2dsuspect%2dbcg%2dvaccine%2dafter%2dmany%2dchildren%2dfell
%2dill%2dat%2dschool-name_page.html
Gareth Morgan, Western Mail
PARENTS of a schoolboy who was put into intensive care after receiving the routine BCG vaccination, say they are not satisfied with claims that the vaccine was not faulty. Dominic Hamer, 13, suffered anaphylactic shock - a severe allergic reaction - after the anti- tuberculosis jab was administered at Ysgol Gyfun Penweddig in Aberystwyth last November. There was speculation at the time of the incident that other unwell pupils merely panicked when Dominic fell genuinely ill.
The local NHS trust has now admitted the incident was more than just "hysteria". But in a letter to parents it also insisted there was nothing wrong with the storage, transport or administration of the BCG vaccine.
Dominic's parents say they do not know where to turn, after the letter insisted the vaccine was definitely safe. Yet it also led to 12 other school pupils being taken to hospital. Paul and Maria Hamer said, "We are not satisfied, but we do not know where to go to get any more answers. "We are not interested in compensation, we just want to know what happened and why." Ceredigion and Mid Wales NHS Trust said its findings are correct and have been confirmed by the Department of Health.
It did note that some of the other pupils' problems were reported before Dominic's attack, making an "anxiety reaction" in response to what happened to their friend unlikely. But it concluded that other pupils suffering symptoms like dizziness, nausea and breathing difficulties were experiencing side effects that were "severe, but normal". Last night Mrs Hamer, a nurse, said, "It seems incredible that so many children were affected so badly, all at once.
"At least they admit now that it was more than just a mass panic attack. "But if it wasn't a panic attack, we certainly do not know what did cause the problems, or how we will find out. "These are supposed to be the experts, but they have not told us much and we do not know who else to ask." His father Paul, a garage mechanic, added, "The trust admits that there wasn't mass hysteria by students and that the problems the students had were normal. "But it's a bit alarming that so many people were affected by BCG at the same time at the same place.
"There's been an investigation and that's it - until something happens again." But Mr Hamer, whose own grandfather died from TB, has always urged parents not to withdraw their children from routine vaccinations. Despite his own son's reaction to the jab, he said that maintaining public immunity to the disease is important. Dominic, from Bow Street,near Aberystwyth, returned to school eight days after his ordeal. He had spent a night in intensive care with breathing difficulties, before being transferred to a general ward. Mrs Hamer said, "He is great now, all the children are fine, which is the most important thing. "It was a short-term effect thankfully and he is now back to playing his rugby again."
Even after his severe reaction to the BCG, the then-Welsh Health Minister Jane Hutt said there were no grounds for suspecting any fault with the vaccine.
Pediatric Dermatology
Volume 22 Issue 2 Page 130 - March 2005
doi:10.1111/j.1525-1470.2005.22208.x
http://www.blackwell-synergy.com/links/doi/10.1111/j.1525-1470.2005.22208.x/abs/
Vaccine-Associated "Wild-Type" Measles
Kelle Liermann Berggren, M.D.*, Michael Tharp, M.D.*, and Kenneth M. Boyer, M.D.
Abstract:
Measles is the most contagious of the childhood exanthems and is the leading cause of vaccine-preventable deaths in children, mostly in developing countries. The prodromal stage, consisting of high fever and the triad of cough, coryza, and conjunctivitis, is followed by a caudal progressing rash over a period of 2 to 3 days. With a worldwide vaccination program in place, mortality and morbidity have decreased substantially. Receipt of the live attenuated vaccine generally causes no or only mild side effects such as a low-grade fever and a subtle rash.
We report a 1-year-old boy who, 10 days after vaccination, developed vaccine measles which was clinically indistinguishable from the natural disease. Vaccine virus was detected by polymerase chain reaction in the patient's nasopharyngeal secretions
Hmmm... looks like the vaccine didn't prevent that vaccine-preventable case!
Flu clinic nurse faces charges
Injections were given in 3 county stores
Mary Lane Gallagher, The Bellingham Herald
http://news.bellinghamherald.com/stories/20050324/TopStories/236471.shtml
Investigators still aren't sure what was in the syringes at flu shot clinics offered at three Lynden and Blaine grocery stores last fall, but prosecutors have charged the nurse who allegedly administered them with practicing medicine without a license.
Whatcom County Prosecutor Dave McEachran charged Nancy Jean Olson with injecting seven people with "a purported flu shot," according to the charging documents. Prosecutors allege Olson injected at least 130 people at grocery stores in Lynden and Blaine in November 2004. Olson, a Canadian, is now in British Columbia, said Detective Lee Beld of the Lynden Police Department. A warrant is out for her arrest, Beld said, and authorities have asked Olson to turn herself in to police in the United States.
Olson was charged with one misdemeanor count and six felony counts of unlicensed practice of medicine. According to charging documents filed Tuesday in Whatcom County Superior Court, Olson held flu shot clinics in November at Cost Cutter and Food Pavilion stores in Lynden and a Cost Cutter store in Blaine. The $30 shots were offered at the height of a nationwide shortage of flu vaccine. Some people who received the shot complained to the store when they didn't get the side effects they expected. Store managers then discovered the nurse administering the shots provided a false company name. Olson later told investigators that she used the name "Pacific Coast Consultants" when working with grocery stores "to make her sound more professional," according to the charging papers.
Investigators determined Olson was the nurse in question after one of her co-workers at Alderwood Convalescent Center in Bellingham spotted her on a television news story about the shots, according to the charging documents. She no longer works in Whatcom County, Beld said. Olson told Lynden police that she had ordered the flu vaccine from the manufacturer Aventis Pasteur, but the company said they had no record of Olson receiving the vaccine or of shipping any to her address.
Then Olson told investigators she had gotten the vaccine from Hoagland's Pharmacy in Bellingham, from a man who had recently died, but according to prosecutors she did not have valid receipts from the pharmacy. "We have gone down the road that she has set before us as to where she says she got the vaccine," Beld said. "We haven't been able to confirm she has ever purchased the vaccine." Nor have investigators concluded what was in the shots, Beld said. Olson gave investigators a half-full vial containing flu vaccine originally sold to Alderwood Convalescent Center, prosecutors said. But tests of empty syringes from a flu shot clinic Olson held at a church in Everson found "a substance not consistent with ... vaccine having been present," according to the charging documents. Some syringes didn't have enough liquid inside to test.
"It's possible that it was saline solution," Beld said, "but we cannot tell for sure." So far, no one has complained of health problems because of the shots, Beld said. "From what the Department of Health has told us, that's a very good sign," he said. Brown and Cole Stores, which owns Cost Cutter and Food Pavilion, sent a letter to customers who said they had received the questionable shots, and offered them legitimate flu vaccine as well as a store gift certificate, said store spokeswoman Sue Cole.
Store managers are also more diligent in checking out non-employees who want to offer health services in stores, she said.
Reach Mary Lane Gallagher at mary.gallagher@bellinghamherald.com or call
715-2285.
50-children fall sick after being vaccinated by polio drops in Jhang
Thursday April 28, 2005 (0502 PST)
http://www.paktribune.com/news/index.php?id=103258
JHANG, April 28 (Online): At least 50 children have been affected by different diseases like cholera, vomiting at the Kashmir Colony, Jhang district after vaccinated by polio drops during polio eradication campaign . The vaccination campaign was launched by the health department Jhang on
April 26, 28 with an objective to eradicate polio from the district. But due to poor quality vaccine 50 children of the Kashmir Colony have been affected by different diseases like Cholera, vomiting after polio teams vaccinated them .
And when parents and school teachers went to the office of Zonal Supervisor Dr. Shahid Bukhari to make a complaint against the grave situation, he become furious and insulted the complainants . After this, the parents rushed towards Executive District Director Officer Health Jhang Dr. Mushtaq Ahmad Shad office carrying a written application but he was not absent from the office . They have called upon government to probe the matter as it is matter of life and death of children who are future of the nation.
End.
Dubious vaccination sickens 120 pupils
www.chinaview.cn 2005-06-25 20:00:23
A six-year-old girl died and at least 121 school children became sick after getting dubious vaccinations in eastern China's Anhui Province, a hospital source said Saturday.
HEFEI, June 25 (Xinhuanet) -- A six-year-old girl died and at least 121 school children became sick after getting dubious vaccinations in eastern China's Anhui Province, a hospital source said Saturday. Without approval from local health and education departments, the epidemic prevention center in Dazhuang Town of Sixian County inoculated bacterin against hepatitis A to more than 2,500 children in 19 primary and middle schools on June 16 and 17.
The girl named Li Wei died in a local hospital Thursday afternoon after contracting fever, diarrhea and twitch. Experts of the provincial sanitation department said at the end of an initial probe that the girl died of serious infection and respiration failure. Legal medical experts from the provincial medical institute dissected her body Friday afternoon but no outcome have been available so far. To console the girl's parents and other close relatives, the relevant local government departments in the county have reached an agreement to pay the girl's family l90,000 yuan (some 10,840 USdollars) in compensation.
Other school kids, aged about seven to 14, are still undergo ingintensive medical care in hospital and 20 of them remain in critical condition, according to doctors. Pan Longgen, the physician-in-charge with the Sixian People's Hospital, said the ailing students' hearts and livers have been harmed to varying extent. But so far, there has been no effective way for treatment, he added.
The bacterin has been sent to the Chinese Ministry of Public Health for testing and the outcome is expected to be available in about 10 days. Enditem
Vaccine incident moral: No room for compromise http://www.chinadaily.com.cn/english/doc/2005-07/02/content_456511.htm
Yan XizaoChina Daily Updated: 2005-07-02 06:58As we get closer to the truth behind the vaccine accident in Sixian County, Anhui Province, the messier things get. Finding the facts appears trickier than we could have imagined. Hundreds of students in a Sixian township have shown unusual symptoms after taking a hepatitis A vaccine the local epidemic prevention agency purchased from a private wholesaler. An affirmative conclusion about the nature and cause of the incident is still pending. There simply were too many variables with potential to cause trouble. We hope the laboratory tests now under way might finally corroborate the judgment of experts that this is purely a rare case of collective hysteria.
However, such an outcome might prove disturbing, because it could discount people's sense of urgency to clear up the mess. All legal requirements about authorization and procedures, as stipulated in the new Regulation on Vaccine Circulation And Inoculation, must be satisfied. Even if violations are not found to be the direct cause of the incident, they could well become the cause of some other incident on another day. There is no room for compromise. Duely followed procedures and authorizations are a matter of life or death in this regard. Beyond that, as the entire picture unfolds, we see it as an imperative to re-evaluate the state of epidemic prevention and control network.
A Beijing News investigation found no clues indicating that a hepatitis A outbreak exists in the region. And some of the students involved had taken a hepatitis vaccine in the recent past and already enjoyed immunity from the disease. In that case, a collective approach to inoculation was unnecessary. An official with the local epidemic prevention station confessed to the newspaper the station had not submitted an application for the inoculation because it knew it would not be approved. But they provided the shots, anyway, because it was a paid service. They bought the vaccine at 4.5 yuan (55 US cents) per shot from a wholesaler, and charged students 25 yuan (US$3) for each injection.
Twenty-five yuan is not a small amount in a place where the farmers' average annual income is a mere 2,000 yuan (US$240). Imposing such an unnecessary financial burden on poor rural households is immoral. The way it was done was illegal. But it was not done without a reason. Epidemic prevention workers wanted money to support themselves. Only four of the six members of the station are covered by the local financial budget. They receive just 150 yuan (US$18) a month.
This is reminiscent of the well-known problems in our discourse of farmers' financial burdens. It offers fresh proof for the popular understanding that overstaffed township administrations constitute an unbearable financial burden on farmers. The outrageous price of the shots - 25 yuan for a 4.5 yuan wholesale cost says it all. Still, we need well-grounded evidence to attribute this malpractice at the township epidemic control station to redundancy. The SARS outbreak two years ago taught us epidemic control was an essential though dange rously weak link in our public health system.
It still is.
The embarrassment of the township epidemic prevention station in Sixian County shows either there are too many people on its payroll, or they are underfinanced. And the way they handled the vaccine inoculations showed they lacked professional training, either in expertise, or in morality.
We need to decide upon reasonable and affordable personnel levels for the epidemic prevention network, so that people can be properly trained and paid to do their jobs. State and local governments share an obligation to provide adequate financial support for epidemic prevention, considering its impacts on people's livelihoods. It is a shame when those who are supposed to serve the public welfare become a threat to it.
http://www.newsday.com/news/local/wire/newjersey/ny-bc-nj--dyfsoffice-death1020oct20,0,1210809,print.story?coll=ny-region-apnewjersey Infant dies after being stricken at DYFS office October 20, 2005, 6:19 PM EDT
NEWARK, N.J. -- An infant died Thursday after being stricken at a downtown office of the state child welfare agency, police said.
The month-old boy, who has been in foster care since birth, had just arrived about noon at an office of the state Division of Youth and Family Services from a doctor's visit. He had received immunizations for hepatitis B and polio at the doctor's, said DYFS spokesman Andy Williams.
"When the aide arrived to bring the baby upstairs, he noticed he was having trouble breathing and there was blood in his nose," Williams said.
The aide brought the child to the office nurse, who began resuscitation efforts while emergency medical workers were called. The first-aid crew took the baby to Saint Michael's Hospital, where he was pronounced dead at 12:46 p.m., Williams said.
"It appears the baby had a reaction to an immunization," Williams said.
An autopsy is to be done to determine the cause of death, police said.
The child, whose name was not released, was placed in foster care immediately after being born Sept. 16, Williams said.
The foster mother had taken the infant to the doctor's. After the visit, a DYFS driver took the boy to the DYFS office, where the infant was scheduled to have a supervised visit with his natural mother, Williams said.
Bereavement counseling is being offered, Williams said.
Copyright 2005 Newsday Inc.
http://www.nytimes.com/2005/10/23/weekinreview/23pollack.html
Lessons From a Plague That Wasn't
By ANDREW POLLACK
Published: October 23, 2005
PUBLIC health experts warn that the world might be close to a repeat of the flu pandemic of 1918, which killed millions. But could it instead be close to a reprise of the 1976 pandemic that never happened?
That is the year President Gerald R. Ford announced a crash program to "inoculate every man, woman and child in the United States" against swine flu. But the virus never became a killer, and vaccinations were halted two months after they began after reports that 500 people who received the shot developed a paralyzing nerve disease and more than 30 of them died.
A vaccination in Watsonville, Calif., in 1976 as part of a crash program to fight swine flu.
If scientists were wrong in 1976, could they be wrong now? Some arguments being made about why a pandemic is looming echo those made three decades ago. But many experts say the situation now is different enough that a false alarm is less likely.
"We just know a lot more about the influenza virus than we did in 1976," said Ira M. Longini Jr., a professor at Emory University who is an expert on epidemics. Still, a lot can be learned both from what did and did not happen back then. The 1976 scare started in February when a handful of soldiers at Fort Dix in New Jersey got sick and one of them died. Scientists determined that the virus was one that infected pigs and was different from the human influenza viruses circulating then. On March 24, barely a month later, President Ford announced the vaccination plan.
One reason for the concern was that scientists thought the 1918 pandemic had been caused by a swine virus and that the Fort Dix outbreak marked its second coming. Furthermore, experts warned that pandemics tended to be cyclical and that another one was about due. Today, thanks to genetic analysis - a technique not available in 1976 - scientists know the 1918 virus was a bird virus that mutated. So now there is concern that the H5N1 avian strain ravaging birds in Asia could in like fashion evolve into a form that can spread easily among people. The avian virus shows some mutations similar to those in the 1918 virus, said Jeffery K. Taubenberger of the Armed Forces Institute of Pathology. And experts are again warning that the world is overdue for a pandemic.
Edwin M. Kilbourne, a professor emeritus at New York Medical College who argued for the vaccination program in 1976, said there was actually less reason to be concerned about a pandemic today. That is because the swine flu virus at Fort Dix clearly passed easily from
person to person, while the current avian flu has not. Many experts disagree, however. In retrospect, they say, the 1976 decision to vaccinate was based on much less solid evidence than is available today.
"Part of the problem was convictions outpacing evidence," said Harvey V. Fineberg, president of the Institute of Medicine, part of the National Academies, and co-author of "The Epidemic That Never Was," a book about the 1976 experience. "I don't think that's
happening today."
Even in 1976 there seemed to be more doubt than there is today about the issue. The World Health Organization and many European nations, for instance, did not see what the fuss was about. Today, they are busily preparing for a pandemic. Doubts grew through the summer of 1976 when the virus was not detected outside Fort Dix. That first death turned out to be the only known one from the virus. Moreover, studies suggested that several hundred people at Fort Dix had been infected but most never got sick.
"That's very different from 120 cases with half the humans dying," said Dr. Fineberg, referring to the approximate toll so far from the Asian bird flu.
Perhaps the biggest mistake in 1976 was giving the vaccine to millions of people rather than just stockpiling it until clearer signs of an epidemic emerged. W. Paul Glezen, a professor at Baylor College of Medicine in Houston, said the prevailing belief was "we should use it because if it started spreading there wouldn't be time." The vaccination program had problems. Production was delayed after one company produced the wrong vaccine. Another delay occurred when manufacturers demanded that Congress protect them from liability. Mass inoculations did not start until October, which might have been
too late had a pandemic begun.
Still, by the time the program was halted in December, only 10 months after the virus was identified, about 150 million doses had been made and 45 million given. There are lessons from 1976 that might be applied to today's preparations. A 2004 draft of a government plan for a pandemic listed some. One is that "making clear what is not known is as important as
stating what is known." Another is that a program should be re-evaluated periodically rather than left on autopilot.
And this time, federal officials said, the vaccine won't be used until there are signs a pandemic is under way. Dr. Fineberg said another lesson is that Congress should provide liability protection for vaccine makers now, rather than waiting until the crisis occurs. He said the wrong lesson to draw from 1976 would be "the superficially obvious one" - that because it didn't happen then it won't happen now and preparations are not necessary.
Still, repeated cries of wolf can make the public blasé.
"There's so much expectation for it to develop into a pandemic that if it does not in the next year or two it's quite possible you would see a backlash like the 1976 experience," said Dr. Taubenberger. "What I fear is that people would make the conclusion, falsely, that influenza is not such an important public health problem."
[PROVE Note: Does anybody really know what is in those vials?]
http://www.chron.com/cs/CDA/ssistory.mpl/front/3422308
Oct. 28, 2005, 8:51AM
1,000 get possibly fake flu vaccine
By ARMANDO VILLAFRANCA
Copyright 2005 Houston Chronicle
More than 1,000 Exxon Mobil employees received flu shots last week with a vaccine now being investigated by the FBI to determine its authenticity. The U.S. Food and Drug Administration is testing samples of the vaccine. The flu shots were given to about 1,000 Exxon Mobil employees and 80 contractors during a Health Fair Oct. 19-20 at the company's Baytown complex of refinery and chemical plants.
Jeanne Miller, a spokeswoman for Exxon Mobil in Baytown, said a state-licensed independent physician's office was contracted to administer the shots. Miller would not identify the office. In the past, she said, a company medical team administered the flu shots to employees at that plant.
She said the FBI notified Exxon Mobil at its corporate offices earlier this week that it was investigating the authenticity of the vaccine. Miller did not know whether the investigation had been ongoing or had stemmed from the use of the vaccine at the Baytown complex.
The FBI in Houston referred all comment to the U.S. Attorney's Office.
In a statement released Thursday, U.S. Attorney Chuck Rosenberg said a preliminary investigation does not show the vaccine to be harmful, but measures were being taken to ensure its safety. "Those known to have received the fake flu vaccine have been notified and steps are being taken to ensure their well being," he stated. "We are also attempting to ascertain whether others might have received a fake vaccine."
If a fake flu vaccine was used, he said, those responsible for its use will be prosecuted for fraud. Miller said all 3,800 employees at the Baytown complex and contractors were notified Wednesday. "We have not received any reports of illness potentially related to the vaccine," she said. She said Exxon Mobil also recommended that employees given the flu shot should be tested for blood-borne pathogens, or germs.
armando.villafranca@chron.com
Vaccine blamed in avian flu outbreak 11/10/2005
asahi.com:Vaccine blamed in avian flu outbreak - ENGLISH The Asahi Shimbun
The agriculture ministry suspects an unauthorized vaccine from Central America caused the avian flu outbreak in Ibaraki Prefecture that could have been spread by farm employees, journalists and the transport of chicken droppings, sources said Wednesday.
The Ministry of Agriculture, Forestry and Fisheries said it was highly unlikely that a migratory bird or imported pet brought the H5N2-type virus to the prefecture in the summer, according to a draft interim report on the issue.
The full text of the report was to be released today.
The first case of avian flu was detected at a chicken farm in Mitsukaido, Ibaraki Prefecture, in June. Chickens at 34 farms mainly in the prefecture were later found infected with the same strain of the virus.
The interim report deals with 31 of the 34 farms.
The report noted that the DNA sequence of the H5N2-type virus was "almost identical to that confirmed in Guatemala and other countries in Central America."
The ministry said an unauthorized bird-flu vaccine originating in Central America might have been brought into Japan. Instead of immunizing the birds, the vaccine infected them, the sources said.
The report said this theory is backed by the fact that the areas of infection were limited mainly to southern Ibaraki Prefecture.
A U.S. expert's opinion is also included in the report. The expert says the Central America-originated vaccination might have been brought into Japan through a Southeast Asian country hard hit by the highly virulent avian flu.
The ministry will conduct further investigations to pinpoint the source of infection, as well as any use of unauthorized vaccines.
According to ministry officials, the spread of the virus might have been caused when chicken droppings from one infected farm was transported to others in Mitsukaido for compost and other purposes.
The report cited four other possible routes: poultry farm employees who visited shops or other places and left behind the virus; reporters traveling from infected farms; indirect contact between chicken-farm employees and customers at egg-selling stands; and the transport of chickens.
Reporters had visited a poultry farm in Bando in Ibaraki Prefecture just after the Mitsukaido case came to light. Genes of the avian flu virus were also confirmed at the Bando farm, the report said. (IHT/Asahi: November 10,2005)
http://www.cbs13.com/topstories/local_story_075122330.html
CA Company Recalls Measles Vaccines Sold Overseas
(AP) EMERYVILLE, Calif. The Bay Area biotech company linked to recent flu shot shortages said Thursday that it was recalling and withdrawing a measles mumps and rubella vaccine it supplies to developing countries and Italy because of a higher rate of adverse effects than similar vaccines.
Chiron Corp. said about 5 million doses of the Morupar vaccine were distributed to developing countries in 2005, and about 450,000 doses were distributed in Italy. The company said the side-effects included fever, allergic reactions and glandular swelling, which occurred just after inoculation. Chiron said in a statement the reactions do not indicate any long-term risk and that the recall and withdrawal were a precaution. The recall does not affect its other vaccines, Chiron said.
Emeryville-based Chiron set off a public health panic in 2004 when it failed to deliver half the nation's expected 100 million flu shots after British regulators discovered contaminated vaccine at its Liverpool, England, manufacturing plant.
Last fall, Chiron Corp. again said it wouldn't be shipping as many flu shots as it initially had hoped, saying production delays and decreased output occurred after it made repairs at its Liverpool plant. Chiron has said it delivered 13 million flu vaccine shots in 2005, well below the 18 million to 26 million doses it had initially planned to make. Chiron said it will work closely with the World Health Organization to conduct a risk-benefit analysis to see if the measles, mumps and rubella vaccine should be rereleased in limited quantities.
The recall prompted the company to lower its fourth-quarter results by 3 cents per share.
The company revised its earnings to $138 million, or 68 cents per share, from $144 million, or 71 cents a share, for the fourth-quarter, and to $180 million, or 94 cents per share, from $187 million, or 97 cents a share, for the year. Chiron reported results in late January. The Morupar vaccine booked $10 million in sales for 2005. Chiron wrote off about $6 million of Morupar inventory for 2005 and recorded $1.7 million of reserves for anticipated product returns. Shares of Chiron fell 6 cents to $45.64 in midday trading on the Nasdaq Stock Market. The Federal Trade Commission has approved the company's acceptance of a $45-per-share buyout offer from Swiss drug maker Novartis AG.
Novartis already owns 44 percent of Chiron and has offered $5.1 billion in cash for the rest of the stock. The two companies hope to close the deal in the first half of this year, but four shareholders owning a combined 17.5 percent of the company oppose the deal as too cheap. One of those shareholders, ValueAct Capital, said Wednesday it will call for the ouster of Chiron Chief Executive Howard Pien if he backs Novartis' bid and the shareholders turn it down.
"If Howard Pien is going 'on the road' to argue for the appropriateness of the Novartis offer, we can only conclude that our confidence in him has been betrayed, and that he has chosen not to accept responsibility for maximizing shareholder value," ValueAct partner G. Mason Morfit wrote in a letter to Pien and the Chiron board.
(© 2006 The Associated Press. All Rights Reserved. This material may not be published, broadcast, rewritten, or redistributed
REUTERS Health
SOURCE: Morbidity and Mortality Weekly Report, February 23, 2006.
Mumps outbreak at camp among immunized children
NEW YORK (Reuters Health) - In 2005, an outbreak of mumps occurred in a New York summer camp despite the fact that all of the children involved had adequate vaccination coverage, according to the Morbidity and Mortality Weekly Report.
The camp, which had 541 staff members and campers, was in session between June 28 and August 18. Thirty-one cases of mumps were identified, for a 5.7 percent attack rate, Dr. K. Henry, from the Sullivan County Health Department, and associates report.
The first case was in a 20-year-old unvaccinated man who arrived from the UK, where a mumps epidemic was ongoing, to work as a counselor at the camp. His first symptoms, which were seen on June 30, included inflammation of the left salivary gland, sore throat and low-grade fever. However, he continued to work among the camp population.
Between July 15 and July 23, twenty-five more cases of swollen salivary glands were identified, but no diagnoses of mumps were made until July 24. Twelve cases were among campers between 10 and 15 years old, all of whom had been vaccinated with two doses of measles-mumps-rubella (MMR) vaccine after their first birthday. The 19 cases among staff members included nine individuals from the UK, five from the US, three from Australia, and two from Germany. Vaccination was documented for only eight of those affected. Henry's group notes that symptoms, illness duration, and complications were the same for vaccinated and unvaccinated individuals. Although generally a mild and self-limited viral infection, mumps can lead to meningitis, encephalitis, pancreatitis, deafness, inflammation of the testes, and spontaneous abortions among pregnant women.
Therefore, the authors recommend that camp counselors be vaccinated against vaccine-preventable diseases such as mumps. "To prevent large outbreaks of mumps in their communities," the investigators recommend that "US health-care providers should suspect mumps independent of vaccination history, diagnose mumps by using laboratory testing, and report mumps immediately to local health authorities."
Thanh Nien News | Health | Vietnam orders ban after UK-made children vaccine proves deadly
& ASSOCIATED PRESS
Published on 8/28/2005
Cikeusal, Indonesia— Holding her 2-year-old son, Sari listens intently in a ramshackle health clinic as the medical staff assures her and other villagers about the safety of the vaccine being used to fight Indonesia's first polio outbreak in a decade.
But the impoverished mother of two remains unconvinced. She hints she will not participate in Tuesday's nationwide immunization campaign because of unfounded rumors that a neighbor's child contracted polio after being given the oral vaccine earlier this year.
“I'm afraid. Maybe my boy will get paralyzed,” said Sari, who was among 62 percent of parents in her village who refused to get their children vaccinated in June during a regional campaign on Java, the main island where most of the nation's 226 polio cases have occurred.
Such fears are threatening the biggest public health exercise ever mounted in Indonesia, whose rising caseload has the World Health Organization worried that the virus could spread throughout Southeast Asia.
Indonesian leaders are pulling out all stops to win over a public skeptical about the drive to vaccinate 24 million children under age 5 on Tuesday and then again on Sept. 27.
The two largest Muslim organizations in the world's most populous Islamic nation are endorsing vaccinations in TV ads, and busloads of soap opera stars and singers are making the rounds to promote a $24 million campaign comparable in preparation to a general election.
More than 750,000 vaccinators will be on hand Tuesday at 245,000 posts set up at health clinics, bus depots, rail stations and airports. The army and police will help deliver vaccine — by plane, boat, bicycle and foot — to some of Indonesia's 6,000 inhabited islands.
“The biggest challenge is public trust,” said UNICEF's Claire Hajaj, who works on the U.N. agency's global campaign to eradicate polio in the six countries where it remains endemic, as well as in Indonesia and 16 other nations that recently have been re-infected.
“The key is that community fears get addressed and they don't turn into widespread vaccine avoidance,” Hajaj said.
A 20-month-old diagnosed with polio in March was the country's first case since 1995. Authorities believe the child caught it from a migrant worker or tourist who was infected in Africa or the Middle East.
Polio spreads when unvaccinated people come into contact with the feces of those with the virus, often through contaminated water in places with poor hygiene or inadequate sewage systems. It attacks the nervous system in children under 5, causing paralysis, muscular atrophy and sometimes death, although only about one in 200 of those infected ever develops symptoms.
The potential for the virus to spread beyond Indonesia's 210 million people has prompted East Timor, the Philippines and Thailand to launch smaller vaccination campaigns.
“If this virus continues to spread, we are talking potentially hundreds more Indonesians becoming paralyzed,” said Arun Thapa, who is overseeing WHO's polio eradication campaign in Southeast Asia and visited Indonesia this past week.
Indonesia's polio outbreak first prompted authorities to vaccinate as many as 6.5 million children in Java province during two rounds earlier this year.
But officials missed 1 million children in the second round after parents were scared off by false media reports that three children died from taking vaccine or rumors that the vaccine violates Islamic law.
The rumors mirrored those that spread across the West African nation of Nigeria in 2003, where polio vaccinations were suspended for several months after radical Islamic preachers told parents they were dangerous and part of a U.S. plot against Muslims.
Islamic leaders in Indonesia sought to put such rumors to rest by issuing a fatwa saying the vaccine does not violate Muslim dietary law. But in provinces like West Java, which has 58 polio cases, that and other rumors persist.
Confusion also lingers among health workers over basic policies, such as whether sick children can be vaccinated. UNICEF says they can.
“Everything is going well, but we are still worried people won't take the vaccine,” said Dr. Agus Gusmara, who heads the campaign in Serang district. “We have still have bad memories of the last two rounds.”
© The Day Publishing Co., 2005
For home delivery, please call 1-866-846-9099
Asheville mother wants answers after 15-year-old son dies suddenly
http://www.citizen-times.com/cache/article/news/71621.shtml By Rebeccah Cantley-Falk, STAFF WRITER Dec. 1, 2004 2:22 p.m. ASHEVILLE - An Asheville mother is desperately seeking answers after her 15-year-old son died Tuesday shortly after receiving a vaccine for Hepatitis B.
Lori Watkins said her son, Kennie Ray Nelson Jr., collapsed after receiving the vaccine at Asheville Children's Medical Center. Nelson then went into convulsions and died around 11:30 a.m., Watkins said. Nelson was pronounced dead Tuesday at Mission Hospitals, spokeswoman Merrell Gregory said. Bonnie Smith, practice manager at Asheville Children's Medical Center, said she could not comment.
"We can't say anything at this point and time," she said.
Hepatitis B is a serious disease caused by a virus that attacks the liver. The virus can cause lifelong infection, cirrhosis (scarring) of the liver, liver cancer, liver failure and death.
Watkins had taken her son for a physical and to get his vaccinations updated in preparation for an outdoors camp he planned to attend. She was waiting Wednesday afternoon for a report from the Medical Examiner to find out what exactly happened to him.
"I was doing what I thought I was supposed to do for my son," Watkins said. "I was trying to protect him from something that could make him sick. And then, the vaccine itself ends up killing him?"
Check back at www.CITIZEN-TIMES.com for more breaking news updates. See Thursday's Citizen-Times newspaper for more on this story.
Families Raise Concern Over Mercury In Vaccines
Debate Continues Over Past Use Of Thimerosal
POSTED: 1:37 p.m. EST November 4, 2002
11:08 p.m. EST November 4, 2002 DURHAM COUNTY, N.C.
A growing contingent of parents believes a mercury-based preservative in those vaccines may have done more harm than good. In 1999, at the request of the Food and Drug Administration, drug companies agreed to begin removing a controversial preservative called thimerosal from vaccines. Some families believe the removal comes too late. Jackson Bono is a happy, curious 13-year-old challenged by a myriad of medical and developmental problems. Jackson has trouble speaking and focusing and works with a tutor.
"The toll it takes on a family is remarkable," said Scott Bono, Jackson's father. Like most parents, Scott and Laura Bono had their son vaccinated when he was a baby. They now blame his problems on thimerosal and its main ingredient, mercury.
"Little did we ever suspect that the very immunizations that were to protect him from childhood diseases were poisoning him with mercury," Scott Bono said. Thimerosal kills harmful bacteria and has been in vaccines for decades. In the early 1990s, the number of recommended childhood vaccines increased. Over the last decade the national autism rate has risen drastically. In North Carolina, the rate has more than quadrupled, according to the state Department of Public Instruction.
Some people see a connection. If you add up the amount of mercury in baby vaccines with thimerosal, the levels exceed those considered safe for adults by the FDA. The Bonos said Jackson was a normal, healthy baby until he received a bundle of vaccines when he was 16 months old. They said, soon after, he stopped talking and making eye contact. Jackson developed autistic tendencies, like spinning uncontrollably. He also suffered severe allergies, seizures and stomach trouble.
"It was a cruel tragedy that happened with our son," Laura Bono said. Dr. Samuel Katz, chairman emeritus of pediatrics at Duke, is considered one of the foremost authorities on vaccines in the country. He raises doubts that thimerosal ever hurt children. "Whenever we have a problem, we like to know whose fault is it. Unfortunately, vaccines have become an easy target," he said. Katz said, "The evidence to support these claims is lacking." However, in 1999, he recommended drug companies take thimerosal out of vaccines. A 2001 report from the National Institute of Medicine also concluded the evidence does not support the claims. Researchers conceded, "the hypothesis is biologically plausible."
"Given that its mercury and we know that mercury has no beneficial effects, my statement to the FDA was that there's really no reason to use something like thimerosal," said Michael Aschner, a Wake Forest University neurobiologist. Aschner has studied mercury for 20 years. Research from the University of Calgary backs up his work and found mercury can destroy brain cells. Aschner points out that the ethylmercury in thimerosal is different from the damaging methylmercury found in some fish. He feels the issue clearly deserves much more study.
"If you do it in a dish, ethylmercury does cause significant effects, toxic effects. There's no question about it," Ascher said. "But, again, what you have to be careful of is how you translate what you see in a dish into a human being." The biggest obstacle parents of special needs children face in making the thimerosal argument is the fact that millions of children, a vast majority, got the same vaccine and never got sick.
"Why is it that all people who smoke don't get cancer? The body reacts differently to different antagonists," Salisbury attorney Bill Graham said. Graham represents 40 families who believe thimerosal hurt their children. He believes evidence is mounting that federal regulators knew that thimerosal could be harmful long before drug companies felt pressure to remove it from vaccines. A study sanctioned by the Centers for Disease Control and Prevention shows infants immunized with thimerosal vaccines were 2.5 times more likely to develop neurological disorders, but it was never released. Instead, the study continued and the results changed. Graham questions why vaccines were never recalled.
"Do you think that thimerosal vaccines that are potentially harmful could still be out there? They could be. They could be on the shelf right now," Graham said. "I really think the thimerosal issue has become a feeding frenzy. It's like the sharks with blood in the water," Katz said. The Bonos said they do not want blood. They want families like theirs to be heard for Jackson's sake, and others like him. "He's lost his childhood and he may not ever be what he should have been," Laura Bono said. Parents like the Bonos can file claims with the National Vaccine Injury Compensation Program. Because of the debate over thimerosal, the federal government has put all the claims on hold until further studies are completed. There was no recall of thimerosal vaccines, so it is possible some could still be on shelves. Anyone with concerns should talk to their child's pediatrician and ask for thimerosal-free vaccines. Both sides of the debate stress the importance of immunizing children.
Reporter: Cullen Browder
Photographer: Gil Hollingsworth
OnLine Producer: Michelle Singer
http://timesofindia.indiatimes.com/cms.dll/html/comp/articleshow?artid=28006270
Hep-B vaccine drive a farce, claims PEEM
TIMES NEWS NETWORK [ WEDNESDAY, NOVEMBER 13, 2002 02:00:00 AM ]
HYDERABAD: All children suffering from the side-effects of the hepatitis-B vaccination campaign in the state must be compensated by Bill Gates, founder of the Bill and Melinda Gates Foundation. The hepatitis-B campaign in the state is a farce and unnecessary. This was the contention of People for Economical and Effective Medicare (PEEM) chairman Dr P V R Bhaskar Rao at a press conference here on Tuesday.
It was well-known that there are adverse effects of the vaccine. The US Congress, having accepted this, has included the vaccine in the National Vaccine Injury Compensation Programme in August 1998, Dr Rao said. Further, he said in the United States, authorities no longer advocate that all newborns be vaccinated against hepatitis-B unless they are born to infected mothers.
Despite this, the state government has no compensation policy for victims suffering from adverse effects of the vaccine, no registry of the children the vaccine has been administered on, no studies about the effectiveness of the vaccine in the state and no information education and communication (IEC) activities carried out, Dr Rao said.
PEEM representatives also appealed to all parents of children who have received the vaccine to claim for a damage of Rs 25 lakh if the child has any side-effects from the Gates foundation. According to the National Vaccination Information Centre (NVIC) report, the protective effect of the vaccine was still unknown, PEEM vice chairman Dr K Venugopal said. Further, the Federal Drug Authority (FDA) has reported that between 1994 and 1998, a total of 23,000 cases of adverse effects due to the vaccine. The PEEM said Bill Gates, his foundation and the state government were using children of the state as guinea pigs.
WASHINGTON (AP) -- A vaccine that cleared toxic deposits from the brains of laboratory mice also doubled the risk of stroke in the animals, according to a study that may offer a new warning sign about a promising therapy for Alzheimer's disease.
The study, appearing Friday in the journal Science, is the first to detect in animals serious side effects of a proposed vaccine that other research has suggested will halt the progression of Alzheimer's disease. A similar vaccine was tested briefly in humans. Researchers have been intensively studying the idea that the brain-destroying disease could be controlled by removing deposits of a toxic substance called amyloid beta that accumulates in the brains of Alzheimer's disease patients.
While researchers are uncertain if amyloid beta is the cause of Alzheimer's or the result of another mind-destroying process, some earlier animal studies found that a vaccine that prompts the body to remove amyloid beta was able to halt the disease and even restore some brain function. The promising results led an Irish pharmaceutical firm, Elan Corp., to test an amyloid beta vaccine on 360 human patients, but the clinical trial was suspended early this year after 15 patients developed inflammation of tissues in the brain. Elan has since said it would no longer test the original vaccine, but will continue to monitor patients who received it.
Unexpected side effects
Now the new study in Science suggests that a vaccine against amyloid beta may have other problems -- a significant increase of bleeding in the brain. Swiss, German and American researchers used a lab mouse strain that had been genetically manipulated to develop the major symptoms of Alzheimer's disease, including the formation of amyloid beta. The mice were injected with a vaccine that caused their bodies to make antibodies against amyloid beta. After five months, the researchers said there was a 23 percent reduction of the amyloid beta in the test mice compared with animals that did not receive the vaccine.
However, the test mice also had twice the number of cerebral hemorrhages, or bleeding in the brain, compared with mice that did not get the vaccine. Additionally, the researchers found six major blood clots among the immunized mice, versus only one among the control mice. The findings, said co-author Dr. Paul M. Mathews of New York University School of Medicine, suggest that scientists still lack a clear appreciation of the possible side effects of Alzheimer's vaccine therapy. "Up to this point, all of the animal studies have been very promising," said Mathews. "This is the first study to show any serious side effects in mice." Back to the drawing board Mathews said the mouse strain used in his study is a closer mimic to the way Alzheimer's appears in the human brain than animals used in earlier studies. He said the mice had deposits of amyloid beta on blood vessels, a very common characteristic of human patients.
Finding this new side effect, he said, suggests that the human trials of Alzheimer's vaccine "were premature." "We need to move back into animals and sort this out," said Mathews. "We need to develop antibodies (vaccines) that don't cause this problem. Otherwise, I don't think this (type of therapy) will work in humans." Bill Thies, vice president for medical and scientific affairs of the Alzheimer's Association, said the new study "is an interesting paper and something that we should look at carefully."
But he said Alzheimer's vaccine therapy remains promising and research should be pursued vigorously. "We are continuing to unravel some of the nuances of it," said Thies. "Whether it ends up being a useful therapy or not is still an open question, but it has enough promise that a lot of people are still working on it."
http://www.cnn.com/2002/HEALTH/conditions
/11/15/alzheimer.study.ap/index.ht=
West Nile Vaccine- Adverse Reaction
Horse Owner Speaks Out
Before I speak, let me preface this article to make it clearly understood, this information is not a blame or vendetta towards anything or anyone. It also needs to be unmistakably understood that I am not for or against vaccinating horses. My sole intent and/or purpose is to educate and inform the horse owner regarding concerns of their horse(s) health in an effort to assist and/or prevent the tragic misfortune that happened to our gelding, Dartanian. He was one of the principal characters in the book, "The Spiritual Life of Horses." His love, integrity, courage, loyalty and quite often humor, will live on in our hearts through his inspiration and valor.
In mid August we were suggested to vaccinate against West Nile Virus, I simply was not well-educated on the vaccine nor the virus or knew the right questions to ask at the time. With the first shot, three of our horses reacted. Our pregnant mare, our gelding and Dr. Donald Warren's stallion, a boarder, all had the same re-action, their front legs swelled. Within 2 days, our gelding Dartanian foundered, shortly there after he died! These are adverse reactions to a vaccine. The reactions our horses experienced are not soley limited to what we saw. Other adverse reactions stated by Fort Dodge's safety study are: systemic reactions, colic, diarrhea, fever, swellings.
The West Nile Vaccine is new. According to Dr. Charles L. McDaniel from USDA, "this is what is called a conditional vaccine. New vaccines such as this, are granted a one year "conditional" trial period. If safety and efficacy are established, a renewal license for a second year may be granted." The West Nile Vaccine license has been renewed for another year. He also stated "when a new vaccine is released it is sold and provided only through veterinarians for one reason, because the manufacturer needs to monitor the field response." (**more information from Dr. Charles McDaniel regarding conditional licensing)
If any reaction occurs then it is the responsibility of the practicing veterinarian to record it and report it to the manufacturer immediately in case there is a problem with that serial batch of vaccine. In the case of the West Nile Vaccine, the manufacturer is Fort Dodge. I spoke with Dr. Tuttle at Fort Dodge regarding our situation and also explained what I was dealing with afterward. Dr. Tuttle, listened intently to what I described, documented my report and said to me, "Lynette, please accept my greatest apologies for what you have gone through, I am a practicing veterinarian, this information is not falling on deaf ears, I will investigate this immediately."
He also stated, "we urge and encourage people to contact us immediately and report adverse reactions to our vaccines. We subsequently report it to the USDA and investigate the serial numbers of the batches that were being used to see if the batch has been contaminated. If people don't report problems then we don't know." Dr. Tuttle also stated to me, " if there is the slightest indication that the vaccine caused a problem then it needs to be reported to us without delay so that we can investigate and research that batch." If your horse(s) have had a reaction to this vaccine, Dr. Charlie McDaniel, USDA and Dr. Tuttle from Fort Dodge are asking you to contact them and report it. The following is their contact information:
Dr. Charlie McDaniel, USDA,
Email: charles.1.mcdaniel@aphis.usda.gov
Tel# 1-515-232-5785 ext. 146
Also www.usp.org/vprp.htm this is a website where you document what happened. Once the information is received it is therefore reported to the manufacturer. The manufacturer then investigates and researches the problem. If a bad batch of vaccine has made it's way to the public it is then recalled. This is my understanding of recourse from both Dr. McDaniel and Dr. Tuttle.
Dr. Tuttle, Fort Dodge,
Email: info@equinewestnile.com Attn: West Nile Product Manager
Tel# 1-800-533-8536
Alzheimer's treatment makes mice brains bleed Immunization used in halted human trial may weaken blood vessels. 15 November 2002
JOHN WHITFIELD
Alzheimer's attacks blood vessels, as well as brain tissue.
© SPL
Immunizing mice against a condition akin to Alzheimer's disease makes their brains prone to bleeding, researchers have found1. This hints at why cerebral inflammation halted an experimental human vaccine trial early this year. The link between mouse and human symptoms is still unknown. But both probably stem from the effects of immunization on damaged blood vessels, says the study's leader, Mathias Jucker of the University of Basel, Switzerland. "These findings are pretty bad for the vaccine," comments neuroscientist Christian Haass of Ludwig Maximilians University, Munich, Germany. "The bleeding is terrible - it could be deadly."
The results suggest that immunization may be more suited to protecting healthy brains than curing diseased ones. Another possibility would be to screen patients for vulnerable blood vessels before vaccination. But other Alzheimer's experts believe that the different symptoms and side-effects in mice and humans make it impossible to connect this research with the trials. "It would be a giant leap to apply this to an Alzheimer's patient," says Roger Nitsch of the University of Zurich, Switzerland.
This is the first time that bleeding has been seen in any animal or human test of Alzheimer's therapies. "We've never seen anything like this - and we've looked hard for it," comments Dale Schenk, head of research at Elan Pharmaceuticals of South San Francisco, the company behind the trial vaccine. The mice that Elan use to study Alzheimer's do not have damaged blood vessels, Jucker counters - so one would not expect immunization to affect them in this way.
Despite the setback, Alzheimer's researchers are still optimistic about the prospects for immunization. Most agree that more results are needed from humans for us to truly understand immunization's effects. Follow-up studies and post mortems of the 375 people immunized in the aborted trial should still give us invaluable information. Beta test The brains of Alzheimer's sufferers contain deposits of a protein called amyloid beta. These are thought to relate to the brain damage and dementia that are symptomatic of the disease. Three years ago, researchers at Elan proposed that injecting amyloid beta into the blood triggers an immune response that fights the disease. No one is yet sure how this works.
Tests were spectacular in mice engineered to develop a form of Alzheimer's. Amyloid deposits shrank, and the animals' memories improved. The vaccine was made from a synthetic version of amyloid. But trials in Alzheimer's sufferers were halted in January, when some patients developed symptoms similar to meningitis and encephalitis. The new finding suggests how this may have come about.
It would be a giant leap to apply this to an Alzheimer's patient Roger Nitsch University of Zurich
Jucker and his colleagues injected elderly mice with antibodies against amyloid, rather than amyloid itself. Five months later, the mice had smaller deposits of the protein in their brains. But they also had many small haemorrhages in cerebral blood vessels. The mice - and most human Alzheimer's patients - have amyloid deposits in the brain's blood vessels, as well as its tissue. Clearing out the protein might weaken these vessels. Vaccinating people before they get Alzheimer's, or in the very early stages of the disease, might help, as amyloid would not have had time to build up, says Richard Harvey, research director of Britain's Alzheimer's Society.
But if immunized human patients bled, it is surprising that none of them had a stroke, Harvey adds. "I doubt that the bleeding is the whole story". Bleeding may be a second side-effect to put alongside inflammation, agrees neuroscientist Dave Morgan of the University of South Florida. <http://www.nature.com/nsu/slices/spacer_trans.gif
References
* Pfeifer, M. et al. Cerebral hemorrhage after passive anti-AB immunotherapy.
Science, 298, 1379, (2002). |Homepage|
© Nature News Service / Macmillan Magazines Ltd 2002
http://www.cm-life.com/vnews/display.v/ART/2002/10/30/3dbf7da3740a9
Meningitis vaccines recalled
By Amanda Cutler
Central Michigan Life
October 30, 2002
Aventis Pasteur issued a recall of four of its single-dose lots of Menomune vaccine, which protects against four strains of bacterial meningitis. Aventis Pasteur is an international researcher, developer, manufacturer and supplier of vaccines. Affected lots were issued after Jan. 2, 2001. University Health Services, which issues the vaccine, did not receive any of the affected lots, said Sarah Campbell, director of University Health Services.
“As a precaution, the company withdrew not only those four lots, but all of the single-dose vials and we did have some single-dose vials,” she said. The company recommends that anyone who received the vaccine from the recalled and withdrawn lots, and is planning to travel to a high-risk country should contact their health care provider to discuss re-vaccination. “They tested some of the vaccine to see if it was effective at the 6-month and 12-month point,” Campbell said. “In four single-dose lots, they discovered that at the 12-month point, it did not protect against one of the four strains of bacterial meningitis.”
Symptoms associated with serogroup A, the strain of bacteria not protected against, include severe headache, stiff neck, nausea and vomiting, fever, a rash and mental confusion. The disease progresses rapidly, Campbell said. Someone affected by the disease could experience symptoms in the morning and be near death in the evening. “There has only been one case of serogroup A meningitis in the United States in the last 10 years,” she said. “So, the people that would need to be concerned are people that were traveling to other parts of the world where they do have epidemics of the serogroup A meningitis.”
Other people who might be affected are those who work in a laboratory or industrial setting, dealing with the serogroup A bacteria, she said. “It’s fairly rare, but we encourage students to consider the vaccination, because of the fact that it is potentially fatal,” Campbell said. Even when it’s not fatal, there are sometimes very serious complications that include amputations, organ failure and brain damage, she said. “I think its important to note that the vaccine still does provide protection against serogroups C, Y and W-135,” Campbell said. “Those are the strains that have been occurring most commonly in outbreaks on college campuses.” University Health Services is still giving the vaccine with the multiple-dose vials, which provides full protection, she said. Students can contact University Health Services at 774-6599 or visit the Primary Care Suite in Foust Hall 104, if they have concerns.
http://www.wfsb.com/Global/story.asp?S=1025686
COLCHESTER -- State health experts are investigating an outbreak of chicken pox in Colchester. 65 students at the Jack Jackter Elementary School got chicken pox last year. It was one of the largest outbreaks in the state. The Centers for Disease Control is also involved in the investigation. That's because some of the students who got sick were vaccinated against chicken pox. The chicken pox vaccine has a 20-percent failure rate.
http://mdn.mainichi.co.jp/news/20021129p2a00m0dm006000c.html
Group outbreak of deadly measles confirmed
KITAIBARAKI, Ibaraki -- A group of junior high school pupils who fell ill earlier this year have been confirmed as Japan's first group outbreak of a potentially deadly measles virus, health officials said Friday. The 109 pupils from Kitaibaraki who fell ill from February to April were afflicted with a H-1 measles virus of a type that has struck widely in China and South Korea. Over 70 percent of the afflicted pupils had been immunized against measles, yet still fell ill. "We need to look into what caused their immunity to weaken," a spokesman from the Ibaraki Prefectural Government's preventative medicine section said. Kitaibaraki Municipal Government officials said the prefectural government will examine why so many of the 109 pupils fell ill even though they had received shots to prevent them from picking up measles. Usually when measles breaks out in Japan, it is carried by D3 or D5 viruses. The differences between these and the H1 virus are not great, but enough for the H1 virus to counter some defense barriers formed by immunization. (Mainichi Shimbun, Nov. 29, 2002)
http://www.chinapost.com.tw/taiwan/detail.asp?ID=32734&GRP=B
Newborn baby killed,six others injured by mistaken
injection
2002/11/30
The China Post staff
One newborn baby was killed, and six others injured yesterday when a nurse at a Taipei County hospital mistakenly injected them with anesthetics that caused them to fall into comas, health officials said. The nurse at Tucheng's Peicheng Hospital was supposed to give the babies hepatitis B vaccinations, but mistakenly injected them with a muscle-relaxing drug, Atracurium, for patients due to undergo surgery, the Department of Health (DOH) officials said. The babies, who had stopped breathing as a result of the injection, were rushed separately to bigger hospitals in the city and county of Taipei for emergency treatment at about 9 a.m., but one of them was pronounced dead in the afternoon. The other six babies were reported to be in a stable condition, but doctors said they had yet to determine whether they had sustained brain damage due to a lack of oxygen.
"Something's wrong, hurry up," a Peicheng nurse, surnamed Liang, reported a panicking voice from within the baby room while she was not far from room teaching mothers how to take care of their new- born babies at about 800 a.m. Liang said before she realized what had happened, emergency crews were rushing the babies away to other hospitals. DOH Secretary-General Lai Chin-hsiang said prosecutors are now investigating the criminal and civil liabilities in the case. A report of the findings of the investigation will be submitted the medical oversight committee, and the hospital may be punished with a license revocation, Lai said. The 21-year-old nurse who committed the lethal mistake, Huang Ching-hui, was granted NT$250,000 bail after an interrogation by the Taipei District Prosecutors Office.
Peicheng director, Hsu Mu-chuan, admitted to an administrative error on the part of the hospital, partly blaming the new packaging of the hepatitis B vaccination for the mistake. Hsu said the vaccines, used to be stored in one-shot (1 cc) bottles, has recently repacked into 10-cc bottles, which he said were similar to those of the muscle-relaxing drug. "We deeply regret the mistake," said Hsu, adding the hospital would give a preliminary compensation of NT$100,000 to each of the victims' families. An anesthetist, surnamed Lee, admitted that she placed nine bottles of Atracurium in the baby ward's refrigerator that also stored the vaccines. She explained that she thought the Atracurim would come in handy when pregnant mothers needed operations. Maintaining that a warning sign was placed on the bottles, she regretted the mistake.
"Nurses should triple check the drugs before making injections," the Central News Agency quoted Lee as saying. But the county's health bureau chief, Lee Lung-teng, came up with a conspiracy theory, saying human error was "unlikely." He said Atracurium did not need refrigeration, and the drug was contained in 2.5 cc bottles. He said the sizes and labels of the two kinds of containers differed a lot, and it would have been impossible for the nurse to misidentify one for the other, and give seven injections without knowing. He said he did not rule out the possibility of the hospital "being set up." DOH head Twu Shiing-jer said the health authorities would provide the families of the victims with help should there be any litigation against the hospital.
http://www.nytimes.com/2002/02/16/international/16BRAZ.html
Merck Says Tens of Thousands May Need Another Hepatitis Shot
Merck & Company said on Friday that an unknown number of people in as many as 27 nations, including 60 000 youngsters in Brazil, might need new shots to prevent infection with the hepatitis A virus because vaccines they received might have been defective.
A unit of the French pharmaceutical group, Aventis, which sells the vaccine in Europe in a joint venture with Merck, disclosed in December that it was recalling batches of pre-filled syringes because they might not be potent enough to protect against the virus, which is spread by poor sanitation and can cause liver damage. At the time, however, neither Merck nor the Aventis Pasteur unit indicated how many people who had taken possibly faulty batches made between December 1999 and December 2001 would need new vaccines.
Although the batches may have been ineffective in protecting against the virus, Merck said the vaccines were not harmful. Gwen Fisher, a spokeswoman at Merck's headquarters in Whitehouse Station, N.J., said on Friday that the possibly ineffective shots of its hepatitis A vaccine given in the past 2 years include both the VAQTA K vaccine for children and the VAQTA vaccine for adults.
"Merck is offering to pay in most countries for either retesting people to see if they are effectively vaccinated or for revaccinations," said Ms. Fisher, who added that she did not know Merck's potential financial liability. Merck commented on the potential number of people affected by the faulty vaccine in response to inquiries by Reuters following reports by local newspapers in Brazil about the use of the possibly ineffective vaccine in Brazil.
Merck sells the VAQTA K vaccine against hepatitis A for young people in the United States, Latin America, Asia, and parts of Europe. The vaccine is put into syringes in Britain by Evans Vaccines, part of PowderJect Pharmaceuticals. In Brazil, a spokesman for Merck's unit there, Merck Sharp & Dohme, said about 60 000 children and adolescents in the country could need another dose of the preventive drug. Merck said the young Brazilians were among those who took about 117 000 possibly faulty vaccines in the past 2 years, mostly in private clinics in the industrialized states of Sao Paulo, Rio de Janeiro, and Minas Gerais. Each dose of the vaccine was given twice to the Brazilian youths and, although not harmful, it may not be potent enough to prevent the disease, the company said. "The reaction varies from person to person and we cannot guarantee that the doses in the problem lots will be effective," said Marcos Levy, director of corporate affairs for Merck in Brazil. Mr. Levy said oxygenated water had seeped past the seal in pre-filled syringes of the medicine packaged in Britain and also sent to the United States, France, Ireland and Germany. "We recalled that lot and other lots that, although still valid, used the same type of seal on the syringes," Mr. Levy said. He added that the Brazilian clinics that gave the vaccine are contacting patients so that they can return for testing and perhaps a new shot. Unlike hepatitis B or C, hepatitis A is rarely deadly and only severe in about 2 percent of cases.
Manitoulin residents get shots of wrong flu vaccine
http://www.thesudburystar.com/webapp/sitepages/content.asp?contentid=14491&c
atname=Local+News
By Star Staff
Wednesday, November 20, 2002 - 11:00
Local News - As Homer Simpson might say, "Doh!"
The Sudbury and District Health Unit announced Tuesday it has learned that some of last year's influenza vaccine was inadvertently included in this year's shipment from the Ontario Government Pharmacy. As a result, people on Manitoulin Island who have already received their flu shot may have to have a second shot to be fully immunized. Using a prior year's vaccine is safe, but may result in inadequate protection for the strain of flu expected to circulate in the current year, the health unit said in a news release. "The Sudbury and District Health Unit has reviewed its records and identified that two clinics on Manitoulin Island used last year's vaccine," the news release said.
"These individuals have already been contacted directly by the health unit and advised to obtain a second flu shot." The health unit also said it has contacted all health-care providers who received flu vaccines this year. Health-care providers were asked to check their vaccine stock and to review immunization records. The health unit requested that anyone identified as having received last year's vaccine be notified directly by the health-care provider.
"If people were immunized with last year's vaccine, they may be only partially protected from the influenza viruses expected to circulate this year," said Kelly Reilly, manager of clinic services with the Sudbury and District Health Unit. "That is why we recommend re-immunization for anyone who is informed that they received last year's vaccine," Reilly said. "Getting a second flu shot is safe and will ensure that people are appropriately protected against influenza this season." The health unit said it will closely monitor the response to its letter to health-care providers and offer assistance as needed. Hope you enjoyed reading The Sudbury Star online. Click here to order convenient home delivery.
Baby, eight weeks, given MMR vaccine Dec 13 2002
http://icwales.icnetwork.co.uk/0100news/0200wales
/page.cfm?objectid=12451367&method=full&siteid=50082
By Owen Fairclough, PA News
A doctor's surgery was today carrying out an inquiry into how an eight-week-old baby received the controversial MMR jab by mistake. Shannon Whitter was given the mumps, measles and rubella vaccination by a nurse at the Bellevue Medical Centre in Birmingham instead of diphtheria, whooping cough and tetanus. The tot has so far suffered no adverse effects from the wrong jab, which is normally GIVEN to youngsters at 12 months. Critics of MMR claim it can lead to autism and irritable bowel syndrome in young children.
Shannon's father, George Whitter, 43, said: "Fortunately, Shannon seems to be fine. "But we're keen to see the outcome of this inquiry because another child may not have been so lucky." Shannon's mother, Christine Fullen, took Shannon to the surgery for her inoculations on Tuesday. After a nurse carried out the vaccinations, they returned to their home in Acorn Grove, Ladywood, - and Miss Fullen found a message on her answering machine from the surgery reporting the blunder. Shannon was taken to the Diana, Princess of Wales Children's Hospital in Birmingham, where doctors told her parents that she was protected against any adverse effects because her mother's antibodies was still in her system.
Miss Fullen said: "We are really angry about this because it should not have happened in this day and age." Dr Andrew Carson, of the medical centre, said the nurse involved would not be administering any further vaccines. "We are looking further with the practice team into what exactly led to this error and procedures will be reviewed to prevent this from happening again," Dr Carson said. "We deeply regret this incident and the concern this has caused to the family." Dr Carson added that manufacturers of the MMR vaccine advise that it is safe to administer it earlier than 12 months, in the event of an outbreak of measles, for example.
Warning over 'useless' mumps vaccine
Dec 3 2002
http://icberkshire.icnetwork.co.uk/0100news/0400bracknell
/page.cfm?objectid=12422754&method=full&siteid=50102
By Colin George
DOCTORS are warning Bracknell parents to be on the look out for a grey-market mumps vaccine which could be useless against the contagious disease. The single jab imported from the Czech Republic is not used by the NHS, but may have been injected into children at private clinics in the area. And youngsters who received the faulty cure may now have to take the controversial combined measles, mumps and rubella vaccine, which some claim to be linked to autism. Medical experts also believe nearly 6,000 doses of the drug Pavivac - which is not licensed by the Government's Medicines Control Agency - are sitting in surgeries waiting to be used. If the vaccine is not kept at a precise temperature it can break down, leaving children unprotected from a range of symptoms including swollen glands, sore throats and fever.
In extreme cases the infection can go on to cause deafness, viral meningitis and sterility in adults. Medicine safety committee chairman Professor Alasdair Breckenridge said: "There are a number of major questions about the manufacture, testing and storage of the unlicensed vaccine Pavivac which are not answered by the information currently available. "Because of this lack of information we are advising its importation and use should be halted as a precautionary measure, and we have also urgently asked for further information and clarification." racknell GP George Kassianos said children who received the suspect vaccine should be given the controversial MMR jab to ensure they are protected. He said: "It is perfectly safe to repeat the dose. Children or adults who have had a single vaccine previously are either immune and unlikely to suffer side effects, or are not immune and need the vaccine."
Published 12/3/2002 http://www.lsj.com/news/local/021203_vaccine_1a-6a.html
Meningitis vaccine might not halt disease
MSU contacting students who got vaccinated
By Sharon Terlep
Lansing State Journal
EAST LANSING - MSU is sending letters to 2,300 students who received meningitis vaccines, after the manufacturer said the inoculations might not work.The nation's only producer of the adult version of the vaccine says it may not ward off a strain found in certain parts of Africa or in laboratories that study the disease.
Vaccine recall
Some meningitis vaccines made between January 2001 and October 2002 have been recalled. People should consider being revaccinated if they received a vaccine in that time and: Work in a laboratory or industry that exposes them to the meningococcal group A. Travel to parts of the world known at the "meningitis belt." This includes parts of Benin, Burundi, Burkina Faso, Cameroon, Chad, Ethiopia, Gambia, Ghana, Mali, Niger, Nigeria, Rwanda, Senegal, Sudan and Tanzania. People who may need a new vaccine should call the place where they received their first one. Revaccinations will be paid for by the manufacturer. On the Web www.menomune.com/page2.html
Michigan State University has Study Abroad programs in Ghana and Senegal - where people could be at risk. But most MSU students are not at risk for meningitis, which is an inflammation of the lining surrounding the spinal cord and brain. The school is sending the letters as a safeguard. "The reality is there aren't a large number of students who travel to Sub-Saharan Africa," said Kathi Braunlich, communications and planning coordinator at MSU's Olin Health Center. "But we want people to be well informed." The faulty vaccines date to January 2001. They're produced by Aventis Pasteur in Bridgewater, N.J. The firm will pay for revaccinations among people who are at risk, spokesman Len Lavenda said. He wouldn't say how much vaccine the company produces or how much might not work. He said, in some cases, tests by the company revealed the vaccine might not protect against Group A of the meningococcus bacteria - found in a strip of African countries known as the "meningitis belt." The illness affects nearly 3,000 Americans a year, though there's been only one death involving the group A strain in the last decade, according to Aventis. "This is not a safety issue and no one needs to be concerned about it," Lavenda said. The Ingham County Health Department is sending about 50 letters to people who may have received the faulty vaccine, Medical Director Dean Sienko said. Shiawassee Country put out a notice Monday offering free revaccinations to people in the affected groups.
At Central Michigan University in Mount Pleasant, 70 miles north of Lansing, a few students have come in for another vaccine, officials said. Meningitis is an issue on college campuses because students living in close quarters are more likely to become sick. It's spread through intimate or household exposure such as kissing, sharing eating utensils or by secretions from the nose and throat. The bacteria have infected six MSU students in the past five years. Three died.
Contact Sharon Terlep at 377-1066 or sterlep@lsj.com.
New military recruit died of meningitis shortly after being vaccinated for it.
http://story.news.yahoo.com/news?tmpl=story&u=/ibsys/20021224/lo_kgtv/1432569
Officials: Recruit Did Not Die Of Strep A
Tue Dec 24, 1:56 PM ET Add Local - KGTV TheSanDiegoChannel.com to My Yahoo!
A Marine recruit who died Dec. 15 had an overwhelming meningococcal bacteria infection that was different from the streptococcus A that infected 185 other recruits at the Marine Corps Recruit Depot, it was reported Tuesday. No other recruits at the MCRD have shown symptoms or have been diagnosed with a meningococcal infection, Capt. John Malone, medical services director at Naval Medical Center San Diego, told the San Diego Union-Tribune. It was purely chance that the two separate bacterial infections, meningococcal and step A, hit the recruit population at the same time, Malone said.
Other members of Pvt. Miguel Zavala's platoon received a special oral antibiotic the day he died that should safeguard them against the bacteria, Malone said.
Doctors did not give the antibiotic, called levofloxacin, to all 4,500 recruits and depot staff because no one else showed symptoms of the rapidly moving infection that killed Zavala, Malone said. Recruits in other platoons and the public are not at risk because the bacteria is only spread to others in the same living area, Malone said. "You have to be in the same household," Malone told the Union-Tribune. "You don't get it by just walking across the parade ground." All recruits entering MCRD are vaccinated against the meningococcal bacteria, but the vaccine is not always effective, Malone said. One recruit remains in critical condition from the step A-related pneumonia outbreak that struck the depot. More than 126 people were hospitalized with pneumonia, though not all were related to strep A.
On a side note, if I had access to the "phials" or "vials", I'd throw them overboard too.
http://www.guardian.co.uk/uk_news/story/0,3604,879153,00.html
Rebecca Allison
Tuesday January 21, 2003
The Guardian
The Ministry of Defence has launched an internal investigation into how dozens of phials of anthrax vaccine were found washed up on a south coast beach yesterday. The packages of ampoules, which were discovered at West Bay, Dorset, posed no risk to health or the environment, according to the MoD.
"There are ampoules of anthrax in containers on the beach but they are not posing a risk to public health. We are working together with local police to make sure the vaccines are disposed of safely," a spokesman said. Anthrax, a biological agent, is one of the main concerns of the UN biological weapons teams searching Iraq. It is believed that the investigation into the discovery of the ampoules will include looking at whether they came from a warship involved in the recent Navy taskforce deployment to the Gulf headed by HMS Ark Royal. Dorset police said the alarm was raised at 11am by the Bridport harbourmaster after a sighting of unidentified packages on the beaches at West Bay. "The phials were confirmed as anthrax vaccine and we were advised they were sterile and contained no anthrax. The services worked to collect the packages.
"As the work was under way other packages, also found to be a medical substance, were washed up and disposed of in chemical bins," a spokesman said. The MoD confirmed that the packages contained two types of phials. One type contained the anthrax vaccine and the other contained phials of a drug called dimercaprol which acts as an antidote to heavy metal poisoning. The packages consisted of individual sealed ampoules which were packaged in sealed plastic boxes and wrapped in polystyrene inside cardboard boxes, the spokesman confirmed.
He said the batch numbers of the anthrax vaccines had been checked and were found to have been manufactured at the Centre for Applied Microbiological Research at Porton Down. "We haven't been able to search the source of the vaccine, but we can confirm it was issued to the armed forces. There is an internal investigation under way to find out how the ampoules came to be in the water," he added.
Friday, February 7, 2003
1,900 got expired measles vaccine (in 1993)
Friday, February 7, 2003 at 09:30 JST
TOKYO — As many as 1,900 children may have been given shots of a measles-mumps-rubella (MMR) vaccine that had already passed its expiry date during the seven months up to the time such vaccinations were banned April 1993, Kyodo News learned Thursday. The Health and Welfare Ministry, the predecessor of the current Ministry of Health, Labor and Welfare, apparently did not disclose the use of the expired vaccine or report it to a sub-panel of the ministry's Council on Public Health that had been discussing rampant cases of the MMR vaccine's side effects.
A group supporting victims of the vaccine's side effects alleges that the government deliberately covered up the expired vaccine's use. An expired vaccine "will not lose its effectiveness immediately or raise the risk of side effects," a ministry official said, but added, "The use of expired vaccines is naturally a problem. We would like to look into the case to find out why that happened. "The vaccine was introduced in 1989 to protect children from the three diseases in a single shot. Production of the stock used for the MMR vaccine was banned in 1991 after it caused side effects in a large number of children. The expiry date for the vaccine was September 1992.
According to vaccine reports that prefectures submitted to the health ministry at the time and other materials, 1,829 people were given the vaccine in eight prefectures between October 1992 and April 1993. Kumamoto Prefecture saw the largest number of people who had the expired vaccine at 801, followed by Hokkaido at 318. Data gathered by members of the predecessor body of the National Institute of Infectious Diseases show that in Tokyo and Kanagawa, two prefectures with no existing records on MMR vaccine use, a total of 104 people were given the vaccine. In all, 1,933 received the vaccine over the period from October 1992 through April the following year.
The health ministry said, however, that the figure may include people who had the vaccine before September, the month it expired, noting that some reports may have come in late. The ministry recently found an in-house document showing it had received reports on five boys aged between 1 and 4 who developed aseptic meningitis after receiving the expired MMR vaccine, but it had kept the fact concealed. A court ruling is expected in March on a suit filed by people who remain disabled due to the vaccine's side effects and the families who lost their children because of the side effects.
Being open and honest defused a bad blunder
Pulse; Tonbridge; Jan 13, 2003;
Full Text:
Copyright CMP Information Ltd. Jan 13, 2003
MMR vaccine; DPT vaccine
Dr Andrew Carson examines the aftermath of a vaccine given in error
The child health clinic had been much like any other, apart from the fact that the regular nurse was away on an immunisation update course and her place had been taken by another practice nurse. The clinic finished just before evening surgery. Suddenly, the relative tranquillity of this interval was shattered by the appearance of the nurse in a state of some distress, come to inform me that she had inadvertently given an eight-week-old the MMR vaccine in place of the DPT and HiB. Discussion with the nurse and health visitor ensued, and the health visitor agreed to contact the family as soon as possible. I did not believe there was any increased risk to the child from the MMR vaccine being given early, but checked this with public health officials and the manufacturers. Both sources confirmed my initial assessment. Towards the end of evening surgery I heard from the parents, who expressed concern and anger. I apologised on behalf of the practice and reassured them that their child was not at any increased risk from the early administration of the vaccine. These points would need to be reiterated frequently over the coming days, at each contact with the parents.
Opportunity for discussion
I invited the parents to discuss things with me as soon as surgery had finished. The discussion was lengthy and covered their distress and concern over the fact their child had received a controversial vaccine without their consent. I felt my function at that time was to listen and be supportive without being defensive. The family have subsequently said that, although the incident should not have happened, they felt very supported through their anxieties by the actions of the practice team.
I concluded my interview with the parents by informing them about our complaints procedure and by giving them details of how I could be contacted personally at all times over the coming days. I contacted them later that night and early the following morning to check that the baby was well. The following morning the sequence of events was reported to our primary care manager who immediately started a Serious Untoward Incident investigation. This involved interviewing all the parties involved, including the parents, to try to establish the cause of the incident and see what additional safety measures could be put in place before the next clinic. The PCT was also informed early in the day.
By late morning on day two we were informed that a relative of the baby had approached the press, and we were asked for a statement by a daily newspaper. After obtaining consent from the baby's parents, a press release was prepared in conjunction with the PCT. The media agency employed by the PCT was invaluable at this stage, fielding much press attention. Misreporting and misrepresentation made the front page in the local paper that afternoon. For example, it was reported that the baby had been rushed to hospital, which hadn't been the case at all. This was followed by a request for an interview by the local television news. The media agency was again extremely helpful in preparing me for the questions.
Vaccinations as usual
We felt it was important to stress the support we were giving to the baby's family, the nurse involved, and the rest of the practice team. Furthermore, it was crucial to reinforce the importance of parents continuing to allow their children to be vaccinated in the usual way. The incident was, after all, a rare and isolated one that had not put the baby involved at any increased risk.
Subsequent activity revolved around accurate documentation and reporting of events surrounding the incident, as well as implementing new safety procedures and issuing a statement to our patients. The MPS had been involved from an early stage and appeared happy with the way we had handled the situation. We also sought advice on catching up with the DPT vaccine that had not been administered. Finally, the nurse involved had to go through a disciplinary hearing.
It was a frantic few days that involved a great deal of upset for everyone involved. But it was also a valuable learning experience and much good has come out of it. For example, we now plan to colour- code our vaccines for easy identification.
The nurse, along with the rest of the team, behaved with great honesty and integrity once the mistake had been discovered. This cannot be stressed too strongly. One hopes that aggressive press attention will never discourage individuals from admitting their mistakes. I am convinced that our being open and honest helped defuse a difficult situation.
Andrew Carson is a GP in Birmingham
Practice candid over MMR error
Pulse; Tonbridge; Jan 6, 2003;
Full Text:
Copyright CMP Information Ltd. Jan 6, 2003
MMR vaccine; DTP vaccine
Dr Andrew Carson found openness was the best policy when his practice nurse accidentally gave an eight-week-old the MMR vaccine. Dr Carson, a GP in Birmingham, commended the nurse for behaving 'extremely responsibly' by informing them immediately when she realised she had given MMR instead of DTP vaccine. Packaging confusion could be to blame. Dr Carson said: 'Once the vial is taken out of the pack the name of the vaccine is actually obscured by the lot number and expiry date. But that is not to excuse what happened.'
Copyright: CMP Information Ltd.
http://news.scotsman.com/latest.cfm?id=5731551
12:31am (UK)
Private Clinics Botched Children's Vaccines - Report
By Pat Hurst, PA News.
Hundreds of children may have been put at risk after two private clinics botched vaccinations for measles, mumps and rubella, it emerged today. Worried families have been told that single shot immunisations given to toddlers at two private clinics, one in Sheffield and one in Hertfordshire have not been done properly. It means children could pick up infections and may not after all be inoculated against the childhood diseases of measles, mumps and rubella. And the vaccines themselves may have become contaminated, leading to an increased risk of children suffering bacterial infections, experts warned. The parents paid the private clinics for their children to receive single injections of the vaccines. Some parents claim the three-in-one MMR injection delivered by the NHS can cause autism and Crohn's Disease. Instead of getting the NHS recommended MMR vaccines they paid around £70 for the single vaccines, according to the Mail on Sunday.
The clinics are run by Lifeline Care Ltd.
At the clinic held at the Hillsborough Sports Arena in Sheffield, 718 children were given the faulty vaccines, while 295 were given the single dose vaccines at the Elstree Aero-Medical Centre in Hertfordshire. The faulty vaccines were given at both clinics between June and December of last year. Most of the children are toddlers. The problem arose because the clinics changed the normal procedure for making up the vaccines, according to the local NHS trust in Hertfordshire. They began pre-preparing batches of vaccines so more children could be treated which investigators think led to the vaccines not working properly. It means potentially hundreds of children were not then protected from the diseases. The error only came to light after two doctors, who worked at the clinic in Hertfordshire, left and wrote a confidential letter to the local Hertsmere Primary Care Trust, which then investigated.
Dr Joel Bonnet, director of public health at the trust, said, "As a result of the changes in the way the vaccines were made up there is a possibility that the efficacy of the vaccine has been effected, so that children are not as protected as normally they would be. "There is a potential risk, which is why we are recommending parents get the children re-vaccinated with the MMR." Dr Bonnet said he was not aware of any of the children who were not properly vaccinated, subsequently falling ill with measles, mumps or rubella.
The clinics concerned defended their actions.
Dr David Pugh, medical director of Lifeline Care, told the Mail on Sunday they followed "common practice" when making up the vaccines. "During last year we had particularly busy clinics and decided to reconstitute the vaccine in advance," he said. "The vaccines were used within the six-hour time scale recommended. The view of the Department of Public Health was that the potency of the vaccine could not be guaranteed in those circumstances." The two clinics concerned will be investigated by the National Care Standards Commission which is the watchdog for all private medical clinics. The General Medical Council will also investigate what went wrong. The clinics are still in operation but have now reverted to making up the vaccines as recommended by the manufacturer. The Hertsmere Trust has written to all the families of the 1,013 children effected to tell them their children may not be properly protected.
It recommends that all children get the MMR vaccine. Any parents who think their child might be effected can ring NHS Direct on 0845 4647. Also more information on the MMR vaccine is available at the following websites:
www.immunisation.org.uk
www.mmrthefacts.nhs.uk
www.phls.org.uk
I guess monkeys, chicken embryos, aborted fetal tissue, and all that is Ok, but my gawd........not a dog. We all know its political, isn't it. BUT again, NO vaccine is safe, including this one. Its just their hypocrisy.....
Sheri
Mumps vaccine suspended in UK
http://www.praguepost.com/P03/2003/Art/0122/news6.php
Safety group concerned that materials in drug present infection risk
By Mindy Kay Bricker
Staff Writer, The Prague Post
(January 22, 2003)
What is good enough for the Czech Republic is not necessarily good enough for the United Kingdom. That was the message from a group of British medical officials who announced Jan. 16 that the United Kingdom would suspend the importation of Pavivac, a Czech-made mumps vaccine for children.
"There are a number of outstanding questions about the manufacture and testing of the unlicensed vaccine Pavivac that are not answered by the information currently available," said Alasdair Breckenridge, chairman of the Committee on Safety of Medicines (CSM), an independent scientific committee that advises the government on medicines. The committee said it was rejecting the vaccine because kidney cells from dogs are used to make it. The CSM said that using materials of animal origin in humans might present a risk from unknown infections.
"There are no other vaccines in the Department of Health's vaccination program which use this method of manufacture," reads a CSM press release. "As a result, there are a number of additional questions not all of which have been satisfactorily answered." Miroslav Reinhardt, export director for Pavivac-maker Sevapharma, said the vaccine was safe and that his company has been cooperative with UK officials. He said the kidney cells were taken from dogs bred at farms that follow laws regulating such practices.
"We cooperated with the Medicines Control Agency (MCA) intensely -- we provided them with our documentation," he said, "and we hope they will reconsider their decision." The vaccine was not licensed for distribution in the United Kingdom. If a medicine meets the special needs of individual patients, doctors in the United Kingdom are allowed to obtain unlicensed drugs. First, however, the drug must be approved by the MCA, the executive agency of the Department of Health that ensures that all drugs meet the appropriate standards of safety, quality and efficacy.
From June to November, more than 5,000 doses of Pavivac were sent to the United Kingdom. Physicians directly requested the vaccine from Sevapharma. Pavivac first came under fire in the United Kingdom in November, when the CSM suspended importation of the vaccine. At the time, some British doctors criticized the action, calling the suspension "appalling scaremongering." When the vaccine was suspended, Czech health officials said they tried to comply with the British government's demands for more information about Pavivac.
"Nothing [about the demands] was very specific or indicated that the product was substandard," said Milan Smid, a doctor for the Czech Regulatory Authority, the government agency that regulates drugs. The agency reviewed the product, Smid said, and did not find any reason to discontinue or halt production in this country. "We didn't have any indicator to take action against the product here," he said. Reinhardt said that no one who has taken the vaccine, which has been used for 14 years, has experienced any serious adverse reactions. "More than 1.4 million children have been vaccinated with no problem," he said of the single mumps jab used in the Czech Republic.
Smid agreed.
"Through the years of use, we had a normal spectrum of the side effects that is comparable to other vaccines," he said. "There is no reason to take actions against this vaccine." United Kingdom health officials said they were unsure how many of those doses imported into their country were administered to children. The CSM has asked clinics to provide a record of children who received the jab so that any adverse reactions can be quickly and thoroughly investigated. Breckenridge said that Sevapharma provided adequate information that proved Pavivac is effective in protecting children against mumps, as long as the child receives a second mumps vaccine between six to 10 months after the initial shot. Currently, Pavivac is sold only in the Czech Republic and is not licensed in any European Union country. The company also manufactures a single measles vaccine, Movivac. British health officials are investigating its safety, even though the measles vaccine has not been imported into the United Kingdom.
Mindy Kay Bricker's e-mail address is mbricker@praguepost.com
Family in new jabs scare
Feb 12 2003
By Ed Reed, The Huddersfield Daily Examiner
LITTLE Oliver Wilson may have to have more vaccinations after new health fears. The Waterloo toddler faces more injections after concerns that separate measles, mumps and rubella jabs given to babies at a private clinic in Sheffield may not have worked. Lifeline Care, which offered single injections at clinics held in Hillsborough Arena last year, failed to meet standards set down by a national watchdog. Now, Alison Wilson, aged 38, and husband John, 37, of Sunny Mead, do not know if 20-month- old Oliver is protected. Oliver went for a measles jab at the clinic last July and a rubella vaccination in October. His parents, who queued for three hours on their first visit to the clinic, wanted Oliver to have separate jabs after hearing how the triple MMR vaccination has been linked the development of autism and the bowel disorder Crohn's disease in toddlers. Single jabs are not available on the NHS. Mrs Wilson found out about the Lifeline Care clinics on the internet. Each injection cost £75. But this week they heard the vaccine given to their son may not have been properly prepared. Oliver, who is recovering from chickenpox, will now go for a blood test to determine whether the vaccines have worked. Sadly, the test is not foolproof and his concerned parents are unsure as to whether they should repeat the jabs. "You pay and put your trust in these people and then this happens," said a worried Mr Wilson. He and his wife were eager to get Oliver vaccinated, but not with a triple jab. "Our best course of action was to go with the single vaccinations," said Mr Wilson. "When I was a child we got single vaccinations. Parents should be able to say whether they want their children to have triple or single jabs." Lifeline Care goes against normal procedure and uses pre-prepared vaccines at its clinics. Health watchdog the National Care Standards Commission has investigated the Lifeline Care sessions and ordered the company to improve its practices and staff training.
Letters are being sent to the parents of 718 children who received vaccines at the Sheffield clinic between June and December, telling them of the problem. Mr Wilson questioned whether all children were affected, or just those who received a certain batch. He criticised Lifeline Care who, he said, had offered no information to worried parents. "They haven't come out with a statement to say whether it was particular batch. All we know is it's the vaccine distributed between June and December." He never remembered being asked for his address by Lifeline Care. Parents with any concerns about their child's vaccination should contact their GP or NHS Direct on 08457 4647.
Parents told to get babies a second MMR jab
By Ian Lloyd
http://www.thisishertfordshire.co.uk/news/barnet/display.var.696255.
index.parents_told_to_get_babies_a_second_mmr_jab.html
More than 40 children from Barnet are at greater risk of catching measles, mumps or rubella after it emerged their doctor is under investigation for giving them faulty vaccinations. Hertsmere Primary Care Trust (PCT) is urging parents whose children were given the MMR jab by GP Dr David Pugh at the Elstree Aeromedical Centre, Elstree Aerodrome, to get them treated again.
The PCT believes 295 children, including 44 from Barnet, 88 from Hertfordshire and 55 from Middlesex, are at risk after Dr Pugh gave them vaccinations prepared several hours before they were due to be administered against the manufacturer's guidelines. The children were treated between June and December last year. "Some children who have been vaccinated may not be adequately protected against one or more of the diseases," said the PCT's director of public health, Dr Joel Bonnet.
"Due to the way the vaccines were prepared, they may have become contaminated." Such contamination would increase the risk of bacterial infections or side effects to the vaccine, he said. While he could not deny he had prepared vaccinations earlier than he was supposed to, in a letter sent to parents Dr Pugh said he had done nothing wrong. "No parent has ever filed a complaint the only reactions notified are normal reactions to vaccinations and none serious," he wrote.
Investigations into standards at the clinic were started after two former members of staff wrote to Hertsmere PCT claiming vaccines were being prepared incorrectly. The clinic is now being investigated by the National Care Standards Commission (NCSC), which monitors standards at private health practices. The NCSC is also looking into Dr Pugh's claims to have administered 9,000 doses of the unlicensed drug Secretin to 1,500 autistic children.
Dr Pugh has been administering the drug since 1998, although there is no information available about its long-term effects.
http://news.sify.com/cgi-bin/sifynews/news/content/news
_fullstory_v2.jsp?article_oid=12631678&category_oid=-20611&page_no=1
Kids given Insulin instead of Hepatitis B vaccine
Thiruvananthapuram, Feb 20
In a glaring case of medical negligence, about 400 children who were mistakenly administered insulin instead of hepatatis vaccine were rushed to the Medical College Hospital in Thiruvananthapuram from suburban Kalliyur on Wednesday evening. Hospital sources said that the children were kept under observation, though the condition of none of them was stated to be critical. "We continue to receive children from the locality as all the children who took the shot today are being asked to get admitted in the hospital," the sources said. Official sources said the vaccination drive was carried out at the Kalliyur Primary Health Centre, near Peringammala In a glaring case of medical negligence, about 400 children who were mistakenly administered insulin instead of hepatatis vaccine were rushed to the Medical College Hospital in Thiruvananthapuram from suburban Kalliyur on Wednesday evening.
Hospital sources said that the children were kept under observation, though the condition of none of them was stated to be critical. "We continue to receive children from the locality as all the children who took the shot today are being asked to get admitted in the hospital," the sources said. Official sources said the vaccination drive was carried out at the Kalliyur Primary Health Centre, near Peringammala.
http://www.thisislocallondon.co.uk/news/
display.var.702494.Top+Stories.clinic_at_
centre_of_child_jab_scare_is_forced_to_close.html
Clinic at centre of child jab scare is forced to close By Ian Lloyd
The clinic at the centre of an investigation into whether it gave hundreds of children ineffective single inoculations against measles, mumps and rubella has been shut down. Lifeline Care Limited, which runs the Elstree Aeromedical Centre at Elstree Aerodrome, was ordered by the National Care Standards Commission (NCSC) to cease practising from 5pm on Friday last week. The clinic, which offered single jabs to combat measles, mumps and rubella, is not registered with the NCSC and was therefore operating illegally. Hertsmere Primary Care Trust (PCT) and the NCSC are currently investigating single-dose jabs given to children at the centre between June and December last year.
Some 295 children including 40 from Barnet may be affected. Meanwhile, there have been unconfirmed reports that a 19-month-old boy, who had the single jab vaccinations at the centre in September, has caught measles. The boy's mother had been told by doctors at Great Ormond Street Hospital that there was a 'strong possibility' he had contracted the disease. A spokeswoman for the NCSC, which monitors private clinics, said: "We have been told by several parents that their children seemed to have contracted measles. But you do sometimes get a mild form of the disease after the inoculation."
Sharon Gold, of Anthony Road, Borehamwood, was horrified when results of a blood test showed her son, who was treated at the clinic, was not immune to any of the diseases. "I am absolutely in shock," she said. The PCT is urging parents who believe their children may have contracted measles, mumps or rubella after being vaccinated at the clinic to write to Dr Joel Bonnet, Director of Public Health, Hertsmere Primary Care Trust, The Elms Clinic, High Street, Potters Bar, Herts EN6 5DA with full details.
http://www.bangkokpost.com/News/03Mar2003_news19.html
Hospitals given vaccines alert after infant's death
Aphaluck Bhatiasevi
State hospitals have been told to closely monitor any adverse effects from vaccine immunisations, following the death of a four-month-old infant this year. Though investigations did not confirm that the boy's death was due to vaccines, health authorities have not ruled out the possibility. Since 1997, there have been five confirmed deaths of children younger than five due to vaccines, according to the Diseases Control Department.
The investigating team, which published their findings in a recent Weekly Epidemiological Surveillance Report, said that although no other child who received the same vaccines suffered any serious side-effects, there was a possibility that the boy could have died from the vaccines. The boy was healthy when taken for a regular dose of DTP and OPV vaccines for diphtheria, tetanus, pertussis and polio at Tha Rua district hospital in Nakhon Si Thammarat on January 15 at 11.30 am. His mother found him dead at 8am the next day.
He had big red patches on the back, under his skin. Investigating authorities did not rule a vaccine-related death because his mother suffered from syphilis during his birth, for which both were treated for 10 days. From 69 children under five treated for side-effects from immunisation at Tha Rua district hospital between October last year and January 21 this year, 18 had pneumonia, 17 suffered from fever while 14 had diarrhoea. Public health authorities have been told to carefully monitor storage procedures and the use of vaccines to ensure safety for children below five years of age.
IVAX Pharmaceuticals Has Recalled ONXOL injection
(SafetyAlerts) - The Food and Drug Administration (FDA) released the following information.
PRODUCT
ONXOL injection (paclitaxel), 300 mg/50 mL (6mg/mL), 50 mL Multi Dose
Vial, Rx only. Recall # D-161-3.
CODE
M026861 Exp Date 02/04.
RECALLING FIRM/MANUFACTURER
IVAX Pharmaceuticals, Miami, FL, by letter on January 20, 2003. Firm initiated recall ongoing.
REASON
Lack of assurance of sterility: Environment in Class 100 Filling room exceeded the non-viable particulate limit specification.
VOLUME OF PRODUCT IN COMMERCE
1,039 vials.
DISTRIBUTION
Nationwide.
Eli Lilly Has Recalled GEMZAR for Injection
(SafetyAlerts) - The Food and Drug Administration (FDA) released the following information.
PRODUCT
GEMZAR for Injection, (Gemcitabine HCl), 200 mg, For I.V. use only, 10 mL
Sterile Single Use Vial, Lilly, Rx Only, Vial No. 7501. Recall # D-157- 3.
CODE
Lot 6MH15.
RECALLING FIRM/MANUFACTURER
Eli Lilly & Company, Indianapolis, IN, by letter on or about December 20, 2002.
Firm initiated recall ongoing.
REASON
Container Defect: glass in vials.
VOLUME OF PRODUCT IN COMMERCE
31,200 vials.
DISTRIBUTION
Agentina, Brazil, Columbia, Mexico, Taiwan and Venezuela.
[http://www.safetyalerts.com/_includes/general.htm]
Of course, the fact that these people are now being exposed to a double dose of adjuvants, heavy metals and toxic contaminants doesn't rate a word of concern, right?
Immunizations underway after vaccination mishap More than 170 babies and children have so far been re-immunised after a vaccination mishap in Ingham in north-east Queensland. About 400 hundred people have been recalled for immunisations received during the past 10 months, because vaccines were wrongly stored at the Ingham Hospital.
Anna Morgan from the Tropical Public Health Unit says authorities have contacted the parents of all but two of the 220 children involved and says the re-vaccination program should last another fortnight. About 30 adults are yet to be contacted to have their tetanus vaccinations again.
http://www.thisishertfordshire.co.uk/news/barnet/display.
var.703249.index.parents_looking_at_legal_action.html
Parents looking at legal action
By Ian Lloyd
Parents of children treated at the controversial Elstree Aeromedical Centre which offers single jab alternatives to the combined MMR vaccine are contemplating legal action against its director Dr David Pugh. Denise Goldsmith said she had been speaking to solicitors following confirmation that her 19-month-old son Noah contracted measles despite being given a £65 single jab at the clinic in September. Noah is one of 295 children who may have been given a faulty jab at the clinic in Hogg Lane, Elstree, between June and December last year.
The National Care Standards Commission, which closed the clinic down on February 20 for not having a licence to practice, is currently investigating the claim. "I am in the process of speaking to different solicitors and I have had about 100 calls from mothers who have had their children vaccinated at the clinic who all want to take it further," said Mrs Goldsmith, of Mill Hill.
"What happened to Noah was absolutely horrific I wouldn't wish it on anybody. The doctors didn't know if it was measles or not because it is so rare as children are vaccinated. "When he went into hospital with big purple spots they were treating him for meningitis for 48 hours." She added: "We sent Noah to the clinic because we didn't want to do the MMR vaccine. "We didn't want to play Russian roulette with his health and risk having an autistic child or a child with bowel disorder."
Dr Pugh, director of Lifeline Care Ltd, which runs the clinic, said he was unable to comment to the Times Group on the matter as his insurance company stated it would invalidate his medical insurance. A statement to parents from the clinic reads: "We have been in private medical practice for over 17 years and offering single vaccines for over five years. "Dr Pugh has been a GP for over 32 years and there has not been a single complaint filed against him."
18:27 Tuesday 4th March 2003
http://mdn.mainichi.co.jp/news/20030312p2a00m0dm004000c.html
Outdated vaccine injected into 1,000s of kids
Over 2,000 children were immunized a decade ago with unreliable vaccines known for causing side effects and well past their use-by date, the Ministry of Health, Labor and Welfare said Wednesday. Physicians across Japan continued to inject children with vaccines for mumps, measles and rubella even after the ministry had decided they no longer should be used. But ministry officials said there was no danger in them doing so. "They're still effective (vaccines) even if they are about half a year past their use-by date," a ministry spokesman said. Typically, the ministry's plan of action is more like one of inaction.
"Our probe into the situation has left us with the impression that individual physicians didn't pay sufficient care with what they were doing. We will set up a panel of experts and look into the correct and fundamental approach to take regarding immunization," the ministry spokesman said.
Ministry officials said the MMR vaccinations were given to children aged from 1 to 6 during the four years from April 1989. As the vaccinations were producing too many side effects, their maker announced in September 1991 that it would cease producing them. The medicines were said to be effective for one year. Ministry officials looked into how much of the outdated vaccines were used after October 1992, the time the ministry's supplies of the drugs reached the prescribed end of their effectiveness.
Going through documents of the only 10 prefectures still with paperwork from the time, the ministry learned 2,070 children had been injected with the out-of-date vaccines. (Mainichi Shimbun, March 12, 2003
Alzheimer's Vaccine Had Mixed Results
By RANDOLPH E. SCHMID
.c The Associated Press
WASHINGTON (AP) - The experimental vaccine withdrawn from testing after four Alzheimer's patients developed brain swelling seems to have reduced the accumulation of brain plaques associated with the disease but may have caused the dangerous inflammation, researchers report. The vaccine did not show success against another sign of the memory-destroying disease, tangled nerves and microfibers, according to the first autopsy results of one of the affected patients in the clinical trial. The 72-year-old woman died almost two years after first receiving the vaccine in July 2000.
The 360-patient trial was halted in January 2002 and the drug, AN-1792, made by Elan Corp. of Ireland, was withdrawn after the inflammation known as meningoencephalitis was found in four subjects. Doctors later discovered 11 more people with the symptoms. Doctors at the University of Southampton in England report that the autopsy results show that the swelling ``is likely to be a consequence of the immunotherapy.'' The vaccine had shown good results in mice, eliminating with few side effects the brain-damaging plaques called beta amyloid.
The accumulation of plaques associated with Alzheimer's seemed to have been cleared from large areas of the human patient's brain, they said. But they found no reduction in the tangled nerves and microfibers also present in the disease. ``That really couldn't have been predicted from the mouse studies because the mice develop only plaques and not tangles as they age,'' said James A.R. Nicoll, lead researcher on the report, appearing in Monday's online edition of the journal Nature. ``In Alzheimer's disease in general, we really don't know how much it is the plaques or the tangles that contribute to the brain dysfunction and dementia - or even if it is neither directly but that the dementia is due to loss of neurons or synapses from the brain,'' he said. The findings may give researchers a start to answering some of those questions, Nicoll said. ``This is the first time we have been able to separately affect plaques and tangles,'' he said.
Alzheimer's affects about 4 million people in the United States, and the number is expected to climb to 14 million by 2050 as the population ages. The disease was first described in 1906 by German doctor Alois Alzheimer and researchers have long sought a vaccine to combat it. There were high hopes for the vaccine when Elan d began testing, based on the results with mice.
In initial tests, humans had no adverse effects. But because the vaccine works by inducing the immune system to attack the protein that makes up those plaques, some scientists had warned that brain inflammation was a potential serious side effect. After halting the trial, Elan ended development of the drug, though the company said it still hopes to produce a vaccine for Alzheimer's using other approaches.
Bill Thies of the Alzheimer's Foundation said the paper is important because it indicates that the vaccine worked in helping remove plaques. That answers a vital question, whether a human given vaccine will generate enough antibodies to change the plaque concentration. ``Clearly the answer is yes,'' said Thies, foundation vice president for scientific and medical affairs. The autopsy results ``suggest an astonishingly powerful effect of the vaccination'' on removing plaques, added to a commentary by researchers at Massachusetts General Hospital who were not participants in Nicoll's report. But the data do not prove the effectiveness of the vaccine because it remains unknown if the symptoms improve after the drug's use, said the commentary by S.M. Greenberg, B.J. Bacskai and B.T. Hyman.
The woman autopsied had a five-year history of progressively worsening confusion and disorientation. She received her first vaccine in July 2000 and this was repeated after four, 12 and 24 weeks. A fifth injection was given at 36 weeks, using a reformulated vaccine. Six weeks later she suddenly had dizzy spells, drowsiness, unstable walk and fever, deteriorating to the point where tests could not be performed on her. She lived another year but showed no improvement in that time. Nicoll said there is no way to determine if the reformulated vaccine caused her sudden deterioration until other patients in the study have died and their brains are examined.
Nature Medicine: http://www.nature.com/nm
Unusual number of Nisswa School students out with chicken pox
NISSWA -- A higher than usual number of Nisswa Elementary School students may have chicken pox. Erin Suemnick, principal at the school, said 29 families have reported that their child has visible signs of chicken pox. Suemnick said in a letter to parents dated Tuesday: "Many of the Nisswa families are already aware of the unusual number of students that are at home already with chicken pox." Also in the letter, Suemnick wrote that the Brainerd Medical Center is interested in the inordinate number of chicken pox cases at the school. The medical center reported many of the children have already had the chicken pox vaccine. A questionnaire was developed to ask parents whether their children have been affected with chicken pox; if their child has had the immunization; or if the child has already been infected with chicken pox. Suemnick said she encourages families to call their doctor if they have any questions regarding their child's condition.
Date: Wed, 14 May 2003 00:15:04 EDT
From: GeoLith@aol.com
Subject: EuroStudy- No Link Between Unexplained Death and Vaccinations...
news about vaccinations, although it would be more reassuring if they were able to state the cause of death...
LONDON (Reuters Health) Apr 28 - The European Medicines Evaluation Agency said on Monday its scientific committee had found no evidence that combination pediatric vaccines were to blame for a number of sudden unexplained deaths. The Committee for Proprietary Medicinal Products reviewed the safety of Hexavac (Aventis-Pasteur MSD) and Infanrix Hexa (GlaxoSmithKline) after five reports of unexplained deaths in children occurring within 24 hours of vaccination. These vaccines offer millions of children protection against six serious life-threatening infectious diseases--diphtheria, tetanus, poliomyelitis, whooping cough, hepatitis B and Haemophilus influenzae b. "The causes of death remain unexplained and on the basis of available data, it is not possible to establish a cause and effect association with the hexavalent vaccines," the committee said in a statement.
"In several cases, sudden infant death syndrome, viral infection, metabolic disorders, allergic reactions or airway obstruction were plausible, however these could not be definitely proven to be the cause of death." Although a family history of epilepsy or convulsions at an early age was reported in three cases the committee decided that the clinical description of these cases did not provide sufficient evidence to identify this as a possible risk factor. It concluded that the benefits of vaccination far outweighed the possible risks of existing vaccines and that vaccination should be continued according to national vaccination schedules.
George Lithco
For info email us at SkipperVigil@yahoo.com
Shaking Kills: Instead Parents Please Educate and Remember If today is an average day in the United States, 4 children under the age of 5 will die or suffer permanent disabilities when they are shaken by a caregiver. Parents can prevent shaking injuries.
The "SKIPPER" Initiative
was formed by concerned parents to increase SBS awareness in the Hudson Valley and educate parents and caregivers about prevention. We are working with government agencies, local hospitals, day care providers, and community organizations to help parents and caregivers prevent shaking injuries.
http://money.iwon.com/jsp/nw/nwdt_rt.jsp?section=news&news_id=reu-n14382087&feed=reu&date=20030514&cat=INDUSTRY
Merck sued by Fox journalist over faulty vaccine
Wednesday May 14, 6:37 PM EDT
NEW YORK, May 14 (Reuters) - A Fox News journalist who contracted hepatitis A despite being inoculated before an assignment in Afghanistan sued Merck & Co. (MRK), the maker of the vaccine, on Wednesday. Merck recalled batches of ineffective hepatitis A pre-filled syringes in December 2001.
The journalist, Claude Novak, could be one of thousands of people who develop hepatitis A, which causes liver damage, after taking the faulty Merck vaccine. Unlike hepatitis B and C, the disease is rarely deadly. The suit, filed in New York State Supreme Court, claims Merck "negligently manufactured, distributed and sold the recalled vaccine which provided absolutely no protection against the disease," according to James, Hoyer, Newcomer and Smiljanich, the firm representing Novak.
A spokesman for Merck said the company has not seen the lawsuit and does not comment on ongoing litigation. He could not say whether this was the first time Merck has been sued in connection with the faulty vaccine. Last February the company said an unknown number of people in as many as 27 nations may need new shots to protect them against the disease because the
ones they received were faulty. A unit of French pharmaceuticals group Aventis SA, which sells the vaccine in Europe under a joint venture with Merck, said in December 2001 it was recalling batches of pre-filled syringes.
Merck sells a hepatitis A vaccine for adults, called VAQTA and one for children, called VAQTA K.
The recall covered vaccines administered after May 29, 2001.
http://www.reutershealth.com/archive/2003/05/01/eline/links/20030501elin019.
html
CDC reports 103 pregnancies in smallpox vaccinees
"We don't have any reason to believe that the vaccine had a causal relationship" to the miscarriages, Nowak said. HERE WE GO AGAIN. There never ever seems to be a causal relationship between vaccines and any event or disease.
Juvenile sarcoidosis after BCG vaccination
> Genevieve E. N. Osborne, MRCP
> Eleanor Mallon, MRCP [MEDLINE LOOKUP]
> Susan C. Mayou, BSc, FRCP [MEDLINE LOOKUP]
> London, United Kingdom
Abstract
We report the case of a 2-year-old boy with juvenile sarcoidosis, in whom the cutaneous lesions first arose at the site of and soon after a BCG vaccination. Juvenile sarcoidosis is rare, and the pattern of clinical features is distinct from the adult form of sarcoidosis, possibly related to immunologic development. The cause of sarcoidosis is unknown, although there is much interest in the possibility of mycobacterial species operating as antigenic stimuli to initiate the disease. This case suggests that the Mycobacterium bovis present in the BGC vaccination may have been etiologically important in the development of sarcoidosis. (J Am Acad Dermatol 2003;48:S99-102.)
This supplement is made possible through an unrestricted education grant from Stiefel Laboratories to the American Academy of Dermatology. From the Department of Dermatology, Imperial College School of Medicine, Chelsea and Westminster Hospital.
Reprint requests: Dr G. E. N. Osborne, Specialist Registrar in Dermatology, St John's Institute of Dermatology, St Thomas' Hospital, Lambeth Palace Road, London, SE1 7EH, United Kingdom.
Copyright © 2003 by the American Academy of Dermatology, Inc.
0190-9622/2003/$30.00 + 0 doi:10.1067/mjd.2003.158
http://www.reutershealth.com/archive/2003/08/08/professional/links/20030808c
lin010.html
Meningococcal C vaccine may cause relapse of nephrotic syndrome in children
Last Updated: 2003-08-08 12:33:14 -0400 (Reuters Health)
NEW YORK (Reuters Health) - Administration of meningococcal C conjugate vaccine (MCCV) appears to increase the risk of nephrotic syndrome relapse in children, according to a research letter in the August 9th issue of The Lancet.
"The risk of relapse after vaccination might be greater for this group of patients than the risk of meningococcal C infection," Dr. Richard S. Trompeter and colleagues write, "so the decision to vaccinate should be carefully considered." Immunogenic stimuli have been associated with the syndrome, but the effect of vaccination has not been investigated, the British researchers note. Their suspicions were raised when nine children with nephrotic syndrome relapsed after receiving MCCV.
Dr. Trompeter, of Great Ormond Street Children's Hospital NHS Trust in London, and colleagues investigated the relapse rate during the year before and the year after vaccination of children with steroid-sensitive nephrotic syndrome.
Included were 106 patients who received the vaccine, among whom there were 63 relapses in the year before and 96 in the year postvaccination, a relative incidence of 1.52 (p = 0.009). The risk was markedly raised only in the first 6 months after inoculation (relative incidence = 1.84). "From 106 doses of vaccine, there was a risk of one relapse in every four doses given to this population," Dr. Trompeter and colleagues write. They suggest that conjugate vaccines stimulate T cells, "so disturbance of the cytokines by MCCV might have resulted in the cluster of relapses we recorded." Lancet 2003;361:449-450.
http://www.thedailystar.net/2003/09/06/d30906011818.htm
3 babies die after 'measles vaccine'
BSS, Jamalpur
Three infants died while six others fell sick yesterday, having been injected 'measles vaccine' on Thursday at an E PI (Extended Programme of Immunisation) centre at Sabilapur under Melandah upazila. The dead, each aged about 10 months, were Mitu, daughter of Khoka Sheikh, Sumyea, daughter of Shakhawat Hossain, and Abu Bakar, son of Suja Mia of Sabilapur village.
Their bodies were sent to Jamalpur General Hospital for autopsy.
ABM Aminullah Nuri, upazila nirbahi officer (UNO) of Melandah, confirmed the death of three infants, adding that the cause of their deaths is yet to be determined. The UNO said the cause of death will be determined after autopsy. An expert team is expected to arrive here today for further investigation, he added. Meanwhile, six others -- Lima, Rupamani, Nirosha, Swapna, Sahedur and Eshrat--became sick after taking the vaccines and are undergoing treatment in different hospitals. Melandah Health Complex sources said the vaccines were made by Serum Institute of India Ltd. A case was filed with Melandah Police Station. Health Assistant Motiyar Rahman and Family Welfare Assistant Shefali Begum of the EPI centre reportedly went into hiding after the incident.
NEW DECISION ON HEPATITIS C
Wednesday, September 24, 2003
By Mike Murphy
YREKA - My office just received some really good news for veterans suffering with Hepatitis C. The newly created specialty rating team in Cleveland, Ohio known as the "Tiger Team" awarded a Vietnam veteran a service connected disability for Hepatitis C. The decision, which just came out in August of this year, was as a result of the "Jet Injectors" used for inoculations of most service members during the Vietnam Era and after.
As I have written about in previous columns, Vietnam Era veterans have been the fastest growing number of Hepatitis C patients. The biggest mystery has always been why. Many of these veterans belong to no "high risk" group such as homosexuals or IV drug users, and many did not even serve overseas. The only risk group they belong to is being in the military during this era. It appears that a link has finally been established as to the reasons for this.
A research project headed by Lawrence Deyton, MSPH, MD, the Director of Aids/Hepatitis at the United States Department of Veterans Affairs in Washington D.C. said in part, "Anyone who had inoculations with the jet injector is at risk of having Hepatitis C and should be tested." Research indicates that the Hepatitis C virus still exists on medical instruments after cleaning with many solutions. I don't believe that this statement could be any clearer.
The jet injector system has long been suspected of transmitting blood borne pathogens. In veterans groups, many believe that the VA purposely denied veterans Hepatitis C claims for being infected with this virus, to hold treatment costs down and give the VA the ability to deny the claim. There were ridiculous studies released indicating the veterans themselves were at fault due to misconduct in, or after military service, that justified the denials.
I remember, not too long ago, the Agent Orange issue was a similar denial by the government and so was the "Gulf War Syndrome." There were similar ridiculous studies released indicating that there was no proof that Agent Orange made anyone ill. Now we all know better. The government went so far as to state that the "Gulf War Syndrome" was a psychosomatic disorder and did not really exist. Now we know better. And now finally we see the truth regarding Hepatitis C. It would appear that the Emperor has no clothes.
The biggest problem to overcome in this issue is getting the word out to these veterans. Most of us who served during this era can remember the long inoculation lines and blood running freely down many of our arms during these inoculations with the jet injectors. Another problem is that the incubation period for Hepatitis C can be decades long and symptoms may be nonexistent up until the time that the veteran becomes very ill. I am asking that anyone who reads this column, please, spread the word and get tested. You can be tested at any VA facility. If you don't know how to access the VA, call our office in Yreka and we will help you. This is extremely important. Your life and the lives of your loved ones may depend on it.
http://www.eastandard.net/headlines/news05102003003.htm
Twins die after polio vaccine
By Joseph Murimi
Four-month-old twins died on Friday night shortly after they were immunised against polio in Kinangop in Nyandarua district. The twins — James Maina Mburu and Samuel Mwangi Mburu — had earlier in the day been taken to Nandaras Health Centre where they were given the oral vaccine. The twins were not sick when they were taken to the health facility for the routine immunisation by their 30-year-old mother, Scola Muthoni Mburu. Muthoni said that when she returned to her home at the Nandaras Scheme, the children did not show any signs of sickness even as late as 8 pm.
At about 1 am, Muthoni said, she woke up and breastfed them, then went back to sleep. She did not notice anything amiss. However, when she woke up yesterday morning at about 8 am, she was shocked to find both twins dead. She called in neighbours and police. It was suspected the polio dose and other injections that go with it may have caused the death. Their bodies are at the Naivasha District Hospital mortuary where a post-mortem will ascertain the cause of death.
Vaccine Blunder Leaves Hundreds Risking Diseases"
Glasgow Evening Times (Scotland) (www.eveningtimes.co.uk) (11/20/03) P. 6;
Anderson, Deborah
The National Health Service in Lanarkshire, Scotland, is contacting over 200 patients after discovering that a number of vaccinations given at Clarkston Medical Centre used vaccines that had passed their expiration dates. The vaccinations may not provide enough immunity, and a phone help line has been set up for those who think they may have received one of the injections. "We can understand that people will be concerned but can assure them that the risk to their health is minimal," says Public Health Medicine Consultant Dr. David Cromie. Some of the vaccinations may have been given over two years ago, and the vaccines given include all childhood vaccinations as well as tetanus and polio vaccines for adults.
http://news.telegraph.co.uk/news/main.jhtml?view=DETAILS&xml=/news/2003/11/2
1/nvacc21.xml
200 patients are given out-of-date vaccines
By Auslan Cramb, Scottish Correspondent
(Filed: 21/11/2003)
More than 200 people have been immunised with vaccine that was past its expiry date, it was disclosed yesterday. The doctor who made the error also admitted that the vaccines, administered over the past two years, had been stored at the incorrect temperature. Dr Tahira Idrees made the errors at the Clarkston Medical Centre, Airdrie. NHS Lanarkshire said some or all of the vaccines might not have given the correct level of immunity. The vaccines were given to patients mainly for childhood diseases such as polio, diphtheria, tetanus, meningitis C and MMR (measles, mumps and rubella).
The health trust is contacting all patients but said the risk to health was minimal. Dr David Cromie, a consultant in public health medicine, said patients would be invited to discuss their concerns and to attend appointments to receive new vaccinations. "We have prioritized the cases involving children up to seven years old," he said. "There have been no reported cases of childhood illness due to ineffective vaccine. "We are treating this situation seriously and a thorough investigation of the circumstances is under way. "The GP concerned has expressed her regret at the anxiety and inconvenience caused."
Also Monday, Kaiser Permanente of Colorado said more than 50 people may have inadvertently been given a polio or pneumonia vaccine instead of the flu shot. Six people may have been given polio vaccine in Englewood and 50 may have gotten a pneumonia shot in Lakewood, spokeswoman Jacque Montgomery said. Neither vaccine should cause health problems and everyone involved will be offered the flu shot again, she said.
http://www.local6.com/news/2665918/detail.html
http://www.sciencedaily.com/releases/2003/12/031223062104.htm
2003-12-25
Invalid Vaccine Doses Would Cost Millions To Fix
Children who receive some of their vaccine doses too soon may need to be revaccinated, at an extra cost of $10 to $18 million a year, according to a new study. "The cost of revaccinating these children is substantial and may impact parents, physicians and vaccine purchasers," say Shannon Stokley, M.P.H. of the Centers for Disease Control and Prevention and colleagues. Their findings appear in the January issue of the American Journal of Preventive Medicine.
Stokley and colleagues' sample of national immunization records reveals that 10 percent of children received at least one invalid vaccine dose in 2002. Invalid vaccines are doses administered five or more days before the minimum age for the first dose or before the minimum time between doses has elapsed. Invalid doses need to be repeated to ensure that children are adequately protected from disease, according to the researchers.
"It is important that vaccines be administered at an age when a child can develop a proper immune response and before significant exposure to natural infection," Stokley says. Of the 2002 incorrect doses, half were for the hepatitis B vaccine, 19 percent were for diphtheria-tetanus-pertussis, 15 percent for chicken pox, 12 percent for measles and only 4 percent for polio.
The first and final doses in a vaccine series were the ones most likely to be given too early, the researchers found. For instance, all of the incorrect hepatitis B doses were the the third and final dose. First doses made up 98 percent of all invalid polio vaccinations. Children who received vaccinations from multiple healthcare providers and those who were born outside the United States were more likely to get an invalid dose. American Indian, Hispanic and Asian children were also significantly more likely to get an invalid vaccination.
Children with mothers who had completed at least some college were less likely to get incorrect doses than those with mothers who had not completed high school, Stokley and colleagues concluded. The study included immunization data collected from 34,087 parents and 22,958 physicians for children ages 19 to 35 months. This story has been adapted from a news release issued by Center For The Advancement Of Health.
Subject: EU drug watchdog probes Aventis, Glaxo vaccines
http://www.signonsandiego.com/news/
business/biotech/20031202-0529-health-europe-vaccines.html
EU drug watchdog probes Aventis, Glaxo vaccines
window.onerror=function(){clickURL=document.location.href;return true;}
if(!self.clickURL) clickURL=parent.location.href; var js=0.0;js=1.0;js=
1.1;js=1.2;js=1.3;js=1.4;js=1.5;
REUTERS
5:29 a.m. December 2, 2003
LONDON – The European Medicines Evaluation Agency will start an active surveillance programme of so-called hexavalent vaccines early next year following reports of a small number of sudden unexpected deaths in vaccinated children. In a statement on its Web site, the agency said four two-year-olds had died soon after being vaccinated, following the authorisation of Hexavac from Aventis-Pasteur MSD, a joint venture of Aventis SA and Merck & Co Inc, and Infanrix Hexa, made by GlaxoSmithKline Plc, in 2000. These vaccines offer millions of children protection against six life-threatening infectious diseases – diphtheria, tetanus, poliomyelitis, whooping cough, hepatitis B and Haemophilus influenzae b. The agency said statistical analysis on data from Germany, where three children died among more than 700,000 given a booster jab, showed that the number of deaths within 48 hours of Hexavac administration exceeded the expected number.
Experts on the agency's Committee for Proprietary Medicinal Products considered that this raised possible suspicions, though they stressed there was no plausible biological reason to link the deaths with vaccination. However, to find out more, prospective and retrospective studies would be carried out by independent institutions and assessed by regulatory bodies. "The results will be closely monitored so that timely regulatory action can be taken, if necessary," the agency said. In the meantime, it reaffirmed its view that the benefits of vaccination far outweighed the possible risks of existing vaccines and that vaccination should be continued according to national vaccination schedules.
Some Arizonans may have gotten ineffective flu shots
Kerry Fehr-Snyder
The Arizona Republic
Jan. 2, 2004 05:30 PM
More than 20 residents in Maricopa County may have received old or ineffective flu vaccines from an unlicensed provider being investigated by prosecutors in Washington. Another dozen residents in Yavapai County also received questionable vaccines from MedSources Inc., a company owned by Shahid Sheikh, prosecuters said Friday. Sheikh is a registered counselor, not a doctor, and is not authorized to give injections, they said. MedSources also gave flu shots in Nevada, Idaho and possibly California, a spokesman for the Bellevue, Wash. Police Department said. "This kind of thing is a rare aberration," said Dr. Bob England epidemiologist with the Arizona Department of Health Services. "It demonstrates that people will try to run scams when they can."
The state health department provided county health departments in Maricopa and Yavapai with a list of individuals who received flu vaccines from MedSources. Those counties are notifying individuals that their vaccines came from an unauthorized provider, and advising them to check with their doctors about receiving another flu vaccine, especially if they are at high risk for complications from the flu.. "Most people are just taking it in stride," said Doug Hauth, spokesman for the Maricopa County Department of Public Health Serivces.
Investigators believe that MedSources gave consumers outdated or weakened vaccines after one of its workers was told to dilute doses, investigators said. They also found Sheikh had flu vaccine made for last flu season that is ineffective against current flu strains. MedSources gave the vaccines at worksites to about 700 individuals, including at least 31 in Arizona. England said he's not aware of any test to determine whether an individual's flu shot will provide protection against the flu. The state health department says about 500 doses of flu vaccine are available in Arizona. Information:
www.cir.org, 602-263-8856 or 1-800-352-3792.
This article can be found at:
http://www.azcentral.com/news/articles/0102badvaccine02-ON.html
http://news.bbc.co.uk/1/hi/scotland/3352425.stm
Inquiry after error over baby MMR
An investigation has been launched after a baby was accidentally given the controversial MMR injection. The three-month-old girl was supposed to have received a meningitis jab. It is understood the mistake was made at Gorbals Health Centre in Glasgow. A spokeswoman for NHS Greater Glasgow confirmed an apology had been issued to the family. Guidelines state babies should be at least 13 months old before they get the measles, mumps and rubella vaccine.
Public health experts said the incident should not cause any long-term problems for the child. Politicians described the mistake as appalling. Scottish Socialist MSP Tommy Sheridan said: "I was horrified when I heard. This case again highlights the issue of the triple MMR vaccine." Scottish National Party health spokeswoman Shona Robison said that "lessons must be learned" from the mistake.
MMR shots go wrong
By Richard Simcox
WORRIED parents have been told their babies’ MMR jabs could be ineffective after being stored at the wrong temperature. Dozens of mums and dads have been recalled to three clinics after the mix-up affecting several vaccinations. The controversial combined measles, mumps and rubella vaccination has been at the centre of a health scare over possible links with autism.
Now parents fear giving their child a second dose so shortly after the first could be dangerous. Tracey Keyes received a letter from director of public health Dr Angela Bhan saying it “could mean” her 17-month-old son Ben’s MMR vaccination was not fully effective. The 29-year-old, who lives in Mosslea Road, Bromley, with husband, Paul, 35, said: “We were appalled they should tell us this without any offer of testing to see whether the first dose had worked. “Given the current controversy surrounding MMR, this was vague at best. There are endless questions we would like answered before we even consider giving our son another vaccination.” Mrs Keyes said she had been persuaded by government advice not to pay £150 to have the single jabs separately at a private clinic.
In her letter to parents, Dr Bhan apologised for any “anxiety or distress” caused. She wrote: “We are sorry this has happened but want to assure you there is no risk of any harm caused by the vaccination your child received.
“To make sure your child is fully protected, we strongly recommend you come back to the clinic to have your child vaccinated again.” In all, 68 children, given either polio, MMR, whooping cough or haemophilus influenzae b vaccinations at Princes Plain, Biggin Hill and Bromley North clinics have been recalled. Nine received the MMR jab.
Bromley Primary Care Trust, which runs the clinics, says it will wait at least one month before re-vaccinating. Dr Nada Lemic, also a director of public health for the PCT, said: “We have reassured parents there is no risk of harm and no cause for concern. “Repeating the vaccination after one month is safe and evidence exists to show adverse reactions to MMR — such as fever, rash or headache — are fewer after a second dose. “We very much regret the inconvenience and worry this may have caused.” The PCT has replaced the faulty refrigerators, checked all the other clinics in the borough and upped the frequency of routine temperature checks. Parents can call the public health office on 01689 880687 during office hours for more information.
4:22pm Tuesday 26th August 2003
http://www.cnn.com/2004/HEALTH/02/13/smallpox.reut/index.html
Breast-fed baby exposed to smallpox vaccine virus
Friday, February 13, 2004 Posted: 9:08 AM EST (1408 GMT)
ATLANTA, Georgia (Reuters) -- A U.S. soldier's wife who was accidentally exposed to the live virus in the smallpox vaccine likely passed it on to her baby through breast-feeding, according to
military authorities.
The incident, which occurred in May 2003 as the U.S. military was inoculating hundreds of thousands of soldiers against smallpox, is the first documented case of third-hand transmission of vaccinia virus through breast-feeding. Vaccinia is not smallpox, but a pox-like virus related to the rare and deadly virus. Cases of accidental transmission are rare, usually occurring through direct contact with a vaccinated person's unhealed vaccination site.
In a report released on Thursday by the U.S. Centers for Disease Control and Prevention, a team of Army doctors reported that the unidentified mother developed lesions near her nipples about a week after her husband was vaccinated. Although the husband had a "major reaction" to the jab, the couple had continued to sleep together and the wife had not stopped breast- feeding their baby. About two weeks later, lesions appeared on the infant's face and tongue. Lab tests confirmed that mother and daughter had been exposed to vaccinia. Neither had been vaccinated for smallpox.
Military officials are not exactly sure how the wife, who had handled laundry possibly contaminated with vaccinia, was infected. They are, however, fairly certain that the baby got the virus through breast- feeding. The report urged breast-feeding mothers living with people who have been vaccinated against smallpox to be aware of the possibility of exposure to vaccinia and take care not to spread it to their infants.
The Department of Defense, which has confirmed 18 cases of inadvertent vaccinia transmission since December 2002, has been advising vaccinated persons to cover up their vaccination sites, wash their hands regularly and limit contact with infants. The United States ended routine vaccinations for smallpox in 1972, but decided in 2002 to resume them for soldiers and some health-care workers as fears grew that the virus could be used as a weapon by radical groups or countries like Iraq.
Smallpox kills about 30 percent of its victims and scars the remainder for life.
http://abcnews.go.com/wire/World/ap20040224_1427.html
Vaccine Boycott Grows in Northern Nigeria
KADUNA, Nigeria Feb. 24 — Two more states in Nigeria's Islamic north joined a boycott Tuesday of a massive polio immunization campaign, demanding government proof the vaccines don't spread AIDS or sterility as Islamic leaders contend. Nigeria, meanwhile, announced it would be days before it had results of lab tests meant to prove the vaccine's safety too late to change any minds among Muslim leaders in the four states that are blocking the immunization campaign, due to end Thursday.
"This is an opportunity lost," U.N. Children's Fund spokesman Gerrit Beger said, confirming that Niger and Bauchi states had joined the vaccine boycott pending findings of the government-led investigation. Northern Nigeria Islamic leaders say the immunization campaign is part of a U.S. plot to depopulate Muslim northern Nigeria by spreading AIDS or sterilizing agents. Northern states maintain their own lab tests show contaminants in the vaccine.
Fourteen million people live in the four states that have blocked the immunization. The World Health Organization says a polio outbreak spreading from one of the states, Kano, has helped spread polio to seven African nations where it had been eradicated. The outbreak and vaccine ban threaten a 16-year worldwide effort to wipe out polio globally by 2005, WHO says. WHO launched the 10-nation emergency immunization campaign on Monday, sending hundreds of thousands of volunteers door-to-door with vaccine to inoculate 63 million children.
On Tuesday, some Muslim families turned away vaccination teams even in states where the campaign has been allowed. Complicating matters, Nigerian Muslim tradition bans male strangers from entering homes with women and girls, forcing the medical teams to send in girls as young as 14 to carry out the inoculations.
"It is difficult to train these young girls to communicate effectively with parents and vaccinate all children in a methodical way without missing some areas," said Usman Kariko Binawa, a vaccine campaign organizer. Just outside the northern city of Kaduna, in the predominantly Muslim village of Maraban Jos, health workers scrawled check marks with chalk on the mud and tin houses of families accepting vaccinations. They wrote large "R's" on the those who refused, so they wouldn't be bothered again. Binta Abdullahi, a 34-year-old campaign worker in Maraban Jos, came out of the home of one Muslim family that had refused to have their children immunized.
"They want to wait for the results of the examinations," she said, referring to the government investigation. In the nearby village of Barakal Allahu, which is mostly Christian, residents welcomed the teams. "We are eager. We've seen polio and we don't want it for our children," said Stephen Dogo, 74, after his two youngest children were vaccinated. In the same village, one polio victim a young wheelchair-bound man with twisted, skinny legs rode past health workers, expressing his approval with the words "God is Great."
Earlier this month, the Nigerian government sent politicians, scientists and religious leaders abroad to witness the polio vaccine been tested in foreign labs. On Tuesday, the fact-finding team said in a statement it was awaiting test results from labs in India and expected them as late as the end of the month, after the vaccine campaign is over. The Associated Press obtained a copy of the committee's interim report that ruled the vaccines safe. However, it acknowledged the tests showed "trace amounts of estradiol," a form of the female hormone estrogen the vaccine's Muslim detractors claim could cause infertility.
The unsigned four-page document suggested the hormone may have come from calf blood serum it said was sometimes used to help produce the vaccine. WHO officials have repeatedly insisted minute amounts of hormones would be completely harmless, amounting to less than what is found in human breast milk. Nigerian Health Ministry officials could not immediately be reached for comment on the leaked document. Muslims in Nigeria's north have been wary of vaccine campaigns since 1996, when families in Kano state accused New York-based Pfizer Inc. of using an experimental meningitis drug without fully informing of the risks.
The company denied any wrongdoing. A U.S. court dismissed a lawsuit by 20 disabled Nigerians who allegedly took part in the study, but a U.S. appeals court later revived it.
Notice to Readers: Manufacturer's Recall of Human Rabies Vaccine --- April 2, 2004
CDC and the Food and Drug Administration (FDA) have been notified that a recent quality-assurance test of IMOVAX® Rabies Vaccine (Aventis Pasteur, Swiftwater, Pennsylvania) identified the presence of noninactivated Pitman-Moore virus (the attenuated vaccine strain) in a single product lot. IMOVAX® is an inactivated viral vaccine and should not contain live virus. The vaccine lot containing noninactivated virus was not distributed.
As a precautionary measure, Aventis Pasteur initiated a voluntary recall of lot numbers X0667-2, X0667-3, W1419-2, and W1419-3, which were produced during the same period as the lot that contained noninactivated Pitman-Moore virus. These four lots, which were distributed in the United States from September 23, 2003 through April 2, 2004, passed all FDA-approved release tests, including testing to confirm the absence of live virus. These test results suggest that any potential risk to those vaccinated with recalled vaccine is likely to be low. No unusual adverse events associated with the recalled vaccine have been reported.
The manufacturer has indicated that additional lots of recalled vaccine were distributed internationally. These lots also passed all release tests, including testing to confirm the absence of live virus. The manufacturer is working with regulatory authorities to determine lot numbers of vaccine and countries that might have received recalled lots. More information about these internationally distributed lots will be provided as it becomes available.
Aventis Pasteur is providing additional detailed information to all distributors and providers. Health-care providers should contact persons who received recalled vaccine to implement the recommendations outlined in this notice (see Recommendations for Persons Who Received Recalled Vaccine). In addition, persons who know they received rabies vaccine between September 23, 2003, and April 2, 2004, should contact their health-care providers to determine whether they received vaccine from one of the four lots being recalled and, if so, whether they should be treated as outlined below. Vaccine distributors and health-care providers who have any remaining doses of the recalled lots should not use them and should contact Aventis Pasteur regarding their disposition. Information about this recall is available from the Aventis Pasteur Medical Information Services Department, telephone 800-835-3587, or at http://www.vaccineshoppe.com.
All persons who have begun a rabies vaccination series (whether for pre- or postexposure prophylaxis) must complete that vaccination series on time, using nonrecalled vaccine. Information about human rabies prevention based on current recommendations of the Advisory Committee on Immunization Practices (ACIP) is available at
http://www.cdc.gov/mmwr/preview/mmwrhtml/00056176.htm.
Recommendations for Persons Who Received Recalled Rabies Vaccine
Most persons receiving rabies vaccine do so because of exposure to a rabid animal, and treatment is needed to prevent fatal illness. Thus, persons who are receiving postexposure prophylaxis (PEP) must not omit or delay receiving any remaining injections; injections needed to complete the series should use nonrecalled vaccine. Recalled vaccine is considered fully immunogenic, and previously administered doses can be considered a dose in a PEP regimen.
Although unlikely, a theoretical possibility exists that persons who received vaccine from a recalled lot could have been exposed to the noninactivated Pitman-Moore vaccine strain of rabies virus. Even in the event of such an exposure, the timely administration of treatment, as described here, will help to ensure negligible risk to persons who have received vaccine from a recalled lot. Persons who received recalled vaccine should receive treatment equivalent to PEP, similar to published guidelines, as follows:
Persons who were vaccinated with recalled vaccine as part of a course of PEP for a possible rabies exposure. Not previously immune (i.e., persons who had not received at least 3 doses of vaccine at some time before the possible rabies exposure). Persons without prior immunity who have a possible rabies exposure routinely receive a 5-dose postexposure immunization series. If this postexposure series has not already been completed, such persons should complete the full postexposure series, using nonrecalled vaccine to complete the series. Doses that have been administered already as part of the 5-dose series need not be repeated, even if recalled vaccine was used. In addition, if rabies immune globulin (RIG)* was not administered with the first dose of vaccine and it has been <7 days since the first dose of vaccine, RIG should be administered at this time. Once PEP is completed, persons are considered fully vaccinated against both the original rabies exposure and any possible exposure to noninactivated virus in the recalled vaccine.
Previously immune (i.e., persons who had received at least 3 doses of vaccine at some time before the possible rabies exposure). Persons with preexisting immunity (i.e., who have completed a full preexposure or postexposure vaccination series) who then have a possible rabies exposure routinely receive 2 booster doses of rabies vaccine. If one or both doses already were administered using recalled vaccine, such persons should receive 2 more doses, using nonrecalled vaccine. RIG is not recommended. Persons who were vaccinated with recalled vaccine for reasons other than a possible rabies exposure.
Not previously immune (i.e., persons who had not received at least 3 doses of vaccine at some previous time). Persons without prior immunity who received recalled vaccine as part of a 3-dose preexposure vaccination series should receive additional doses using nonrecalled vaccine for a total of 5 doses (dosing intervals should follow the PEP schedule as closely as possible). RIG* is recommended if <7 days have elapsed since administration of the first dose of vaccine. Previously immune (i.e., persons who had received at least 3 doses of vaccine at some previous time). Persons with preexisting immunity (i.e., who have completed a full preexposure or postexposure vaccination series before they received recalled vaccine) who received recalled vaccine as a routine booster dose should receive 2 additional doses of nonrecalled vaccine. RIG is not recommended.
All clinically significant adverse events following receipt of rabies vaccine should be reported to 1) Aventis Pasteur, telephone 800-835-3587 and 2) the Vaccine Adverse Event Reporting System (VAERS) at http://www.vaers.org, or telephone 800-822-7967. Additional information about rabies and its prevention is available from CDC, telephone 404-639-1050, or at http://www.cdc.gov/ncidod/dvrd/rabies.
[PROVE Note: People taking a rabies vaccine now have to worry about being exposed to the fatal live rabies virus. In the manufacturing process, a filter failed. Next time somebody tries to tell you the flu vaccine can't give you the flu, or the IPV vaccine (inactivated polio vaccine) can't give you polio, tell them mistakes happen in manufacturing and send them this one.]
http://www.cnn.com/2004/HEALTH/04/03/vaccine.recall/index.html
Thousands of doses of rabies vaccine recalled
Drug firm: One lot had live virus; 3 called back as precaution
(CNN) -- A vaccine maker is recalling thousands of doses of rabies vaccine after discovering through routine testing that one lot contained live virus that could potentially harm human health.
The lot had not been distributed, Aventis Pasteur spokesman Len Lavenda said in a telephone interview with CNN from the company's U.S. headquarters in Swiftwater, Pennsylvania.
As a precaution, Aventis Pasteur is also recalling three other batches of Imovax that were made at the same time, the Lyon, France-based company said.
The Centers for Disease Control and Prevention said in a statement that the lots being recalled were distributed between September 23 and April 2, and "had passed all FDA-approved release tests, including testing to confirm the absence of live virus," suggesting the risk to anyone who may have received the vaccine is small.
Still, anyone who received rabies vaccine between those dates should contact their health care provider, the CDC dispatch said. No unusual events associated with the recalled vaccine have been reported, it added.
Rabies vaccine is made by killing live rabies virus. Injections of the dead virus stimulate the body's immune system to develop antibodies that kill the live virus.
That process went awry at the company's plant in Lyon, where a bulk vaccine filter failed, Lavenda said. That's a critical step in the production process because the solution that typically kills isolated particles of the live virus may not inactivate large particles of it, he said, adding that the filter has been fixed.
Human rabies is rare in the United States, with only 39 cases diagnosed since 1990. But doctors treat possible exposures aggressively because once symptoms develop, the disease is almost always fatal.
Two groups of people get rabies vaccines: those who have been bitten by an animal that is rabid or suspected of being rabid; and those at risk of being bitten, such as veterinarians, zookeepers and people traveling to regions where rabies is common.
Last year, in the United States, about 18,000 people at risk of being bitten got preventive vaccinations, and another 40,000 got them after being bitten.
The only people who need to do something as a result of the recall are those who received the vaccine preventively, Lavenda said. The others, in addition to getting the vaccine, would have been given immune globulin, which contains antibodies that kill live virus. Aventis will pay for those people to get an injection of immune globulin, he said.
The company is also recalling vaccines that were distributed to 18 other countries, and will be sending its customers information about the recall on Monday, Lavenda said.
http://news.bbc.co.uk/1/hi/england/west_yorkshire/3732523.stm
Apology over 'unwanted' MMR jab
Health chiefs have apologised to the parents of a young girl who was given the MMR jab against their wishes. Three-year-old Stevie Hall received the injection at her nursery school in Leeds - even though her parents signed two forms refusing the vaccine. Tim and Jane Hall are now considering taking legal action against Leeds West Primary Care Trust (PCT).
A spokeswoman for the PCT confirmed the incident, which they said was down to a "regrettable mistake".
Human error
Lilian Dalton, business support manager for the PCT, said: "Leeds West Primary Care Trust is sorry if this incident has caused Mr and Mrs Hall upset or distress. "The PCT believes in patient choice, including choice of immunisation. "However, in this instance it would appear that a member of our front line staff made an honest, yet regrettable, mistake and Mr and Mrs Hall's daughter was given the immunisation without parental consent. "We are currently reviewing our procedures to see if changes need to be made in order to avoid mistakes of this nature in the future."
Stevie was given the jab by a health worker who visited Whingate School in Armley, Leeds, on Monday 17 May. Her parents had signed a second form refusing their consent for the vaccination after the original was misplaced.
MMR controversy
They told the Yorkshire Evening Post they signed a form for polio and Hib vaccines, but said no to the combined measles, mumps and rubella injection. Mr Hall, 43, of Armley, said he did not want to take the risk of his daughter receiving the jab because of his uncertainty over the risks involved. He said he was "livid" after hearing about the mistake. The controversy about the MMR injection has raged since 1998 when The Lancet published research that raised the possibility of a link to autism.
Ten of the doctors who co-authored the study, which never proved a link, have since said there was insufficient evidence to draw that conclusion.
Don't they have a lot of these *accidents*?
~~~~~~~~~~~~~~~~~~~~
http://www.northamptontoday.co.uk/ViewArticle.aspx?SectionI
D=255&ArticleID=798768
Boy gets MMR jab without permission
A YOUNG boy was given an MMR injection at a Daventry medical practice even though his parents had refused the controversial jab. The mother of four-year-old Jack Farmer is angry at the mix-up, for which doctors at the Abbey House Medical Practice have issued an "unreserved" apology. Julia Farmer, of Horncastle Close in Daventry, took Jack to the surgery for injections for diphtheria, tetanus, whooping cough and polio, but he was also given an MMR booster without her consent.
Mrs Farmer, 36, said: "I checked Jack's medical book when we left the surgery and realised he had been given the MMR. They were very apologetic but it's something that can't be undone now." A single MMR jab to immunise against measles, mumps and rubella has been linked, without proof, to the development of autism in children. Like many parents, Mrs Farmer and her husband Angus chose to pay for three separate immunisation jabs for four-year-old Jack and daughter Alice, two. Individual jabs are claimed to reduce the autism risk.
Mrs Farmer said: "I am a nurse and my husband and I made an informed decision about how our children would receive the vaccines. "When something is put into your child you haven't given consent for, it's wrong." Abbey House Medical Practice manager Nigel Brown confirmed a child was given the MMR vaccination without the consent of parents. He added: "This incident is viewed most seriously by the practice and a full investigation is being carried out. "The practice again would like to apologise unreservedly to the family for the incident and new procedures will be introduced to prevent a recurrence of the events."
alyson.fixter@northantsnews.co.uk
An outbreak of hepatitis B associated with jet injections in a weight reduction clinic.
Canter J, Mackey K, Good LS, Roberto RR, Chin J, Bond WW, Alter MJ, Horan JM.
Division of Field Services, Centers for Disease Control, Atlanta, GA 30333.
From January 1984 through November 1985, 31 clinical cases of hepatitis B occurred among attendees of a weight reduction clinic (clinic 1). Before the onset of illness, each case-patient had received a series of injections of human chorionic gonadotropin administered by jet injectors at clinic 1. Clinical history, risk factor assessment, serologic evaluation, and review of clinic injection records were obtained on 287 (84%) of 341 persons who had attended clinic 1 in the first 6 months of 1985. Of this cohort, 21% (60/287) had evidence of acute infection with hepatitis B virus (either documented clinical cases or antibody to hepatitis B core antigen, IgM positive). Of persons who had been given human chorionic gonadotropin at the clinic during the period studied, 24% (57/239) of those receiving human chorionic gonadotropin only by jet injector experienced acute hepatitis B virus infection. None of the 22 persons who had received injections only by syringe experienced hepatitis B virus infection. Stopping the use of the jet injectors on July 2, 1985, at clinic 1, was associated with the termination of this outbreak. This investigation demonstrated that jet injectors can become contaminated with hepatitis B virus and then may be vehicles for its transmission.
PMID: 2393323 [PubMed - indexed for MEDLINE]
http://news.scotsman.com/latest.cfm?id=3031039
Doctor Sacked for Giving Wrong Vaccine to Child
By Martha Linden, PA News
A £90,000-a-year doctor was sacked from his job at a private clinic after he gave a child the wrong vaccine and then attempted to cover up his tracks, a disciplinary hearing was told.
Dr Michael Wetzler, of Berkhamsted, Herts, admitted administering the rubella vaccine instead of the measles vaccine to 23-month-old Bradley Wright when the youngster was brought to him last year at the Breakspear Medical Group, in Hemel Hempstead.
At a hearing of the General Medical Council, Dr Wetzler admitted that once he became aware that he had administered the wrong vaccine, he misled Bradley’s parents, leaving them to believe that he had given the measles dose.
He further admitted falsifying the child’s immunisation records by crossing out the word ‘rubella’ and substituting ‘measles’, and providing the boy’s parents with a leaflet relating to the measles vaccine.
The hearing was told Bradley was brought to the clinic, which specialises in allergies and environmental disorders, on June 20 last year by his parents for a measles immunisation.
Adrian Darbishire, for the GMC, said Dr Wetzler had been 40 minutes late for his appointment with the Wright family. He said the couple’s impression of Dr Wetzler had been one of “rushing through the injection”.
Following the immunisation, Mrs Wright felt unhappy about the way Dr Wetzler had treated them, the committee was told.
They became suspicious that their child had been given the wrong vaccine after consulting Bradley’s child health record and noticing that an entry had been made and then crossed out, the committee heard.
Mr Darbishire said Mr Wright asked Dr Wetzler what he had given Bradley. Dr Wetzler admitted that he had given him rubella in error.
Dr Wetzler later appeared before the directors of the clinic and was interviewed about the incident, the committee was told.
In the interview he admitted lying to the Wrights and falsifying documents, and was dismissed for gross misconduct.
Mr Darbishire said Dr Wetzler had told the bosses he had been asked to do “demeaning work” at the clinic.
Earlier, the committee had been told that Dr Wetzler had been appointed in March last year by the clinic with a view to playing a senior role at the establishment.
He was known to the clinic because of his work with cancer patients in Bristol in the early 1980s. He had applied for a job with them from South Africa, where he had worked for a number of years.
The committee heard that Mrs Wright had been “incredibly surprised” to receive a phone call at home from Dr Wetzler after he was dismissed, in which he had told her he had been “very fed up” on the day he had seen them with their son.
He had been refused leave and had been doing “back-to-back vaccinations” of single-dose MMR jabs at the clinic. He told Mrs Wright he had acted in an “immature manner” in the way he had dealt with the error, but he had intended to put it right at the next appointment.
At that point the doctor had become “emotional and started to cry”, Mr Darbishire said.
In further correspondence with the clinic bosses, Dr Wetzler wrote that he felt he would “die of repetitive needle disease” as a result of administering single-dose MMR vaccines to the children of parents anxious about the possible effects of the combined jab.
He said he hoped to bring his deeper, personal expertise to the post when he joined the Breakspear clinic, but instead had been forced to take part in the “mind-numbing activity” of administering the single doses.
Mr Darbishire said there was no suspicion of any harm being caused to the young patient by Dr Wetzler’s action, and he also noted the doctor’s immediate admission of what he had done when he was confronted by Mr Wright at the clinic.
But he said if Bradley had suffered an adverse reaction then medical treatment would have proceeded on an “false basis”.
The hearing continues.
Researchers accidentally exposed to live anthrax
By PAUL ELIAS
AP Biotechnology Writer
SAN FRANCISCO At least five workers developing an anthrax vaccine at a children's hospital research lab in Oakland were exposed to the deadly bacterium because of a shipping foul up, officials reported Thursday. Officials with the Children's Hospital Oakland Research Institute said none of the researchers have shown symptoms of infection, but are being treated with antibiotics as a precaution.
Hospital spokeswoman Bev Mikalonis said the researchers believed they were working with a dead version of the anthrax bacterium, but were instead shipped live anthrax by the Frederick, Md. lab of the Southern Research Institute, which is headquartered in Birmingham, Ala. Mikalonis said other workers may have also been exposed while the live anthrax was being handled and that federal, state and local officials - including the FBI - are investigating.
The hospital doesn't believe that anyone was infected because researchers used proper procedures and precautions while handling the anthrax shots. The Oakland lab itself is located about one mile from the Children's Hospital and Mikalonis said exposure doesn't pose a threat to the hospital and surrounding community. The researchers are working with dead bacterium to develop an anthrax vaccine that children can use. Anthrax attacks killed five people and sickened 17 others in 2001 in the United States. No one has ever been arrested for the killings, but the attacks spurred development of better vaccines and treatments for anthrax than are currently available.
Mikalonis said the Oakland researchers received and stored a shipment of what they thought to be dead anthrax from the Southern Research Institute about three months ago. Last week, the Oakland researchers began injecting anthrax from the shipment in mice as part of an experiment. On Monday the mice unexpectedly began to die. On Wednesday night, California state health officials confirmed their worst fears: live anthrax was in the syringes. Agents with FBI's bioterrorism unit removed the samples from the lab Wednesday.
Southern Research Institute's Thomas Voss, who is in charge of homeland security and emerging infectious disease, said the nonprofit company is investigating what happened. Voss said it's still unclear whether the institute did ship live anthrax to Oakland. "We aren't totally sure of the sequence of events," Voss said. The Southern Research Institute was founded in 1942 and has two highly secure "hot labs" that store some of the world's most deadliest diseases. Labs and researchers from around the country that need data about those nasty diseases but don't - or can't - handle them contract with the Southern Research Institute to do that work.
Voss said the institute's hot labs in Frederick and Birmingham handle just about every "select agent" listed with the Centers for Disease Control and Prevention in Atlanta. The institute is one of 350 entities registered to handle live anthrax with the CDC. It employs 600 people nationwide and has about $75 million in revenue a year, Voss said. Voss said that while the institute receives many shipments of live diseases, it rarely ships them out. "We receive agents on a routine basis," Voss said. "But on our end, we ship very infrequently. I can't even recall shipping live agents."
The mishap will surely be seized on by critics of the government's effort to combat biological terrorism by paying for the construction or expansion of 18 high-containment labs across the country. Supporters of the building boom said the additional lab space is needed to combat emerging global threats, but critics said such expansion increases the likelihood of accidents like the one Oakland reported Thursday.
From 1998
http://www.ias.org.nz/children_contract_tuberculosis.htm
133 Children Contract Tuberculosis in Southern Kazakhstan Itar Wire Service (02/05/98) Kazakhstan health officials report that 133 children in the city of Zhanatas contracted tuberculosis during a routine fall vaccination last year. Although the children contracted TB as early as last September, local authorities reportedly concealed the incident from national officials as they tried to cure the children on their own.
According to deputy chief of Kazakhstan's sanitary and epidemiological surveillance service Albert Askarov, however, their inability to do so forced them to report the incident several days ago. Askarov said the children were infected because untrained medics accidentally used the wrong, more concentrated formulation of the vaccine for injections.
National and regional health officials are now investigating the situation.
Daily Star
Committed to PEOPLE'S RIGHT TO KNOW
Vol. 5 Num 33 Mon. June 28, 2004
Child dies after vaccination in Khulna
Staff Correspondent
A nine-month-old baby girl in Paikgachha upazila of Khulna district died Thursday morning after vaccination at a public immunisation camp. Our Khulna correspondent said Shahanara of Gopalpur village was taken for diphtheria, tetanus and pertussis (DTP) jab to the camp under Extended Programme of Immunisation (EPI).
The vaccine, under routine EPI programme, was the child's second dose in the last three months and seventh dose from the same vial of the vaccine that has also been put into six other children at the camp.
The health directorate described the death as 'unfortunate' and said no one is to be blamed for the incident.
According to the family sources, Shahanara went into severe convulsions on her way home soon after she received the intra-muscular injection vaccine. As she vomited and her condition deteriorated fast, her parents took her to the local upazila health complex. The attending doctor there declared her dead on arrival.
Sources said Civil Surgeon Dr Hame Jamal visited Paikgachha shortly after the incident and asked the father of the child, Punai Gazi, to bury the girl as quickly as possible without an autopsy. Punai, a day-labourer, was also paid some money to compensate for the two days he has had off work due to the death. When contacted, Director of Primary Health Care (PHC) Dr Mahbubur Rahman said, "We cannot rule out the possibility of the child dying in reaction to the vaccine (DTP). In fact, according to the World Health Organisation, death of one in three million from DTP vaccines is globally accepted and is very unfortunate for the child."
Dr Rahman further explained that all vaccines have 80 percent efficacy. The remaining 20 percent of the vaccines can cause accidents and it is globally accepted. Medical experts say a vaccine, like any medicine, is capable of causing serious problems, such as severe allergic reactions. But the risk of DTP vaccine causing serious harm, or death, is extremely small. Dr Rahman said that EPI officials are investigating the incident. While asked about the removal of the vials manufactured from the same batch, Dr Rahman said, "We can't order removal of the vials made in the same batch because it's troublesome and those vials have already been scattered across the country."
The EPI services in Paikgachha upazila remained suspended, health directorate sources said.
http://www.pennlive.com/news/patriotnews/index.ssf?/
base/news/1090402889100480.xml
New shots await 1,200 who got faulty vaccine
Wednesday, July 21, 2004BY DAVID WENNER
Of The Patriot-News
About 1,200 people vaccinated last year at Hamilton Health Center in Harrisburg need to get the shots again because the vaccine was stored in a faulty refrigerator and may have been ineffective. Those affected, mostly children, were inoculated at the Hamilton clinic at 1821 Fulton St. from February through October 2003. Officials said the vaccines in question pose no health threat.
The vaccines protect against debilitating childhood diseases such as diphtheria, tetanus and pertussis, hepatitis B, and measles, mumps and rubella. Jeannine E. Peterson, CEO of Capital Region Health System @ Hamilton Health Center, said the vaccines were stored in a refrigerator that sometimes chilled items to below freezing and below acceptable temperatures.
She said officials aren't certain whether the vaccines are ineffective. But she stressed the importance of the vaccinations and said Hamilton wants to make sure its clients have adequate protection. Hamilton has received no complaints from those who received the vaccine, she said. "We're not in a panic. This is not an outbreak. This is not something we feel [vaccine recipients] need to be overly concerned about," she said. Alice Gray, director of the Pennsylvania Department of Health's division of immunization, also said the vaccine itself poses no health threat. Revaccinations will be free. The health department provided vaccine for the revaccinations at no charge and offered to provide help in giving the shots, she said.
The health department discovered the problem during a routine inspection about three months ago. It used temperature records to determine the affected vaccine had been dispensed only from February through October, Peterson said. Peterson said it took several months for Hamilton to obtain advice from the department and from the U.S. Centers for Disease Control and Prevention on whether Hamilton should revaccinate people who received the vaccine, and to devise a plan.
Capital Region Health System @ Hamilton Health Center is a federally funded health clinic that serves mostly the poor. One of its missions is to reach out to people who might otherwise go without vaccines. Most of the people who received the potentially ineffective vaccine lived in Dauphin County. Some adults also received the vaccines. Peterson said many of the vaccine recipients move frequently, and it is unlikely that letters will reach all of them.
She said Hamilton is working with schools and day-care centers to find those who might need revaccinated. The letters, mailed yesterday, inform clients they need to return to Hamilton for re-vaccination. Staff will use Hamilton records to determine which vaccines the clients need. Hamilton will expand its hours to accommodate the re-vaccinations, Peterson said.
DAVID WENNER: 255-8172 or dwenner@patriot-news.com
http://www.thisislancashire.co.uk/lancashire/leigh/news/LEIGHNEWS4.html
Wrong son got MMR jab - mum
A YOUNG mum is to lodge an official complaint against her family doctor after a vaccination mix-up claim. Emma Hook, 23, and her partner Ian Whalley have started the procedure rolling after her life-long GP gave her 15-weeks-old son Ben Whalley the MMR vaccination meant for his two-years-old brother, Scott.
They claim the doctor should be struck off the medical register and have sent a letter of complaint to the Health Care Commission in Manchester. Emma, of Devonshire Road, Atherton, says she took her two sons to the surgery last Thursday for Scot to have his MMR jab and baby Ben, who was born prematurely, to have his first injections.
She alleges that after Ben was given his injection she was sent out of the surgery and called back later to be told that he had been given the wrong one. "I went numb, I didn't know what to do. Babies are not supposed to have the MMR jab until they are at least 13 months old. We were advised to take him to Wigan Infirmary and there staff rang the Department of Health to see what might happen. Ben was examined by specialists, who told us we were lucky he was under six months old as his immune system was not fully developed. "He had a very high temperature but thankfully he now seems OK. Had he been two months older what would have happened then?
"I am very angry, how can a doctor mix up two injections and give them to the wrong children? I want to make sure this does not happen to anyone else. "He has been my doctor for 23 years, and this is not the first trouble I have had, but it is the first and last time that Ben will see him. I will change doctors and the children will have their injections then." A spokesman for the surgery refused to comment. A spokesman for the Health Care Commission said information was confidential but the couple would have been advised that to follow the NHS complaints procedure they should first lodge a complaint with the practice manager and the complaints manager at the Primary Care Trust.
http://www.nytimes.com/aponline/national/AP-Tainted-Flu-Vaccine.html
The New York Times
August 27, 2004
A Big Maker of Flu Shots Finds Some Contaminated
By LAWRENCE K. ALTMAN
The Chiron Corporation, a major manufacturer of influenza vaccine, said yesterday that it had detected a contamination problem in its new supply and was delaying release of nearly 50 million doses until scientists could determine the cause. Chiron makes about half the 100-million-dose supply that federal health officials expected to be used this flu season. Its chief executive, Howard Pien, said in a telephone interview that it informed the Food and Drug Administration and the Centers for Disease Control and Prevention about the problem on Wednesday. Other than a relatively small amount sent to distributors in July, Mr. Pien said, the company will probably not begin issuing the vaccine until mid-October, about a month later than expected.
Chiron's disclosure to the government agencies came on the very day the Bush administration released the nation's first plan of preparation and response involving the next pandemic of influenza. In that plan, still in draft form, the government underscores a need to help industry improve the production of influenza vaccines for the ordinary seasonal outbreaks as well as for a pandemic. Dr. Julie L. Gerberding, the director of the disease control centers, said Chiron's contamination problem was "not good news," in part because the usual October flu immunization clinics may need to be postponed until November.
But given what Chiron has told her, Dr. Gerberding said in a phone interview, "at this point we don't anticipate an overall shortage.'' As a result of that expectation, she added, the C.D.C. is not now changing its recommendations as to who should be immunized. There are 185 million Americans who ought to be immunized under this year's recommendations, which, for the first time, advocate giving the vaccine - two doses, in fact - to all infants from 6 months to 23 months. In a usual flu vaccination season, most shots are given by Thanksgiving. But a number of public health experts expressed concern yesterday that the delay caused by the Chiron problem would complicate such efforts, because they require considerable planning. The experts said they worried that Chiron was offering a best case.
"It's a mess," said Dr. William Schaffner, chief of preventive medicine at Vanderbilt University. "This will lead to striking disparities in flu vaccine availability around Your Town, U.S.A.'' "If anything,'' Dr. Schaffner said, "this emphasizes the importance of the pandemic plan and the need for more vaccine manufacturers and new immunization techniques.'' Mr. Pien, Chiron's chief executive, said he did not foresee a widespread problem for his company's product, Fluvirin, because, he said, only 8 of 60 batches tested so far have failed to meet the manufacturer's sterility specifications.
"This should have a minimum impact on the American population," he said. Nevertheless, "no assurances can be given that additional tests on Fluvirin will yield satisfactory results or that Chiron will be able to release Fluvirin this season," the company said in a news release. Chiron (pronounced KY-rohn), based in Emeryville, Calif., outside Oakland, manufactures the vaccine in Liverpool, England. Its quality controls cover a wide range of tests, said Mr. Pien, who declined to specify which of those the eight contaminated Fluvirin batches had failed. He said the contamination was biological in nature and possibly due to human error, but again would not be more specific.
The four million doses in the eight batches will be destroyed, Mr. Pien said. He said that though Chiron shipped the first one million doses of Fluvirin to distributors in July, they had not been distributed to doctors and clinics. Mr. Pien said he thought the company would be able to release the rest of its full complement - from 46 million to 48 million doses - by mid-October. Though that would be about a month later than expected, he said, it would still be in time to meet public health needs for this influenza season. Chiron is manufacturing about 25 percent more vaccine than it produced last season. A second major manufacturer, Aventis, said it was ahead of schedule in producing more than 50 million doses. A spokesman for the company said it was surprised by Chiron's announcement and planned to determine whether Aventis could make more vaccine and, if so, how soon.
A third manufacturer, Medimmune, plans to manufacture from one million to two million doses of its FluMist, an influenza vaccine that is sprayed into the nose.
Denise Grady contributed reporting for this article.
Related Stories:
http://money.cnn.com/2004/08/27/technology/chiron.reut/
The Himalayan Times Online
Printed from www.thehimalayantimes.com
Kids critical after vaccination
Himalayan News Service
Biratnagar, September 27:
A medical team has been sent to Dhungesanghu of Taplejung district, where over three dozen children had taken ill three days after being administered anti-measles vaccine on September 21. The health team included WHO representative Jit Bahadur Tam-ang, one representative each from the Regional Education Directorate and District Public Health (DPH), said DPH, Taplejung. RSS said the condition of ten is reported to be critical. The children had vomited blood and have sores in their mouths, locals said. They were administered the vaccine during first phase of the the National Anti-Measles Vaccination Campaign. Asmita Subba, Puspa Subba, Mamata Maswa and Babita Maswa of Dhungesanghu-1, and Sushant Tamang and Januka Shrestha of Ward Nos 4 and 5 of the same VDC are reported to be critical. The sick children's parents said their children suffered from cough, chest pain, high fever, loss of appetite and vomitted blood after being injected with the anti-measles vaccine. Jagannath Adhikari, headmaster of the Party Primary School in Fatyangre, said 25 students have not come to the school after getting the vaccine.
COPYRIGHT@ 2004 THE HIMALAYAN TIMES PUBLICATION. All rights
reserverd
Claims that kids ill after vaccination ‘false’ Himalayan News Service
Kathmandu, September 28:
A government official said today that the reports of children taken ill after the anti-measles vaccination are false. “The medical team which visited Dhungesanghu in Taplejung has submitted its report saying that the children were only suffering from pharyngitis and common cold,” said Bal Krishna Suvedi, of the immunisation department of Child Health Division. “They claim that the illnesses has nothing to do with the vaccination.” The Eastern Region Health Directorate, that had sent a team to the VDC has issued a press release today saying that none of the children were in serious condition. “Some of the children had been suffering from cough, headache and nausea and upon examination, were found to have pharyngitis and cold. The children are better now.” Suvedi also said that the reports in the media which claim children coughing up and vomiting blood are ‘totally wrong’. No other cases of such complications have been reported yet said the official.
The national anti-measles campaign, which is to be implemented in three phases, targets to vaccinate 9.5 million children, between the ages of nine months to 15 years throughout the country. The first phase, which started on September 21, vaccinated children in the eastern and central region. In the second phase, beginning on January 4, 2005, the children in 34 districts of western, mid-western and far-western regions will be vaccinated. The third phase of vaccination, starting on April 16, 2005, will target the six himalayan districts. Around 1,50,000 children across the country suffer from measles every year. About 2,669 measles-related death was reported in the year 2003. So this anti-measles campaign aims to reduce the deaths by half and bring it down to 1,335 by the year 2005.
http://timesofindia.indiatimes.com/articleshow/874894.cms
Polio virus escaped twice from laboratories
TIMES NEWS NETWORK[ WEDNESDAY, OCTOBER 06, 2004 01:25:10 AM ]
NEW DELHI: The polio eradication exercise is beginning to acquire shades of a science thriller. Not only has the virus escaped twice from laboratories in the country, but also caused the crippling disease, acute flaccid paralysis in 10 persons.
The government has now started a mammoth exercise to gather a list of laboratories storing the polio virus before it can claim to have eradicated it. Contrary to popular knowledge that the polio virus is transmitted only through contaminated food and water, it can also be passed on by an infected person through respiratory secretions, such as coughing and sneezing. Laboratory strains of the virus were identified earlier in three polio-stricken cases in 2000 and another seven more recently.
The government is well aware that the entire eradication effort could be at risk if the virus were to escape from different laboratories. A task force under the chairmanship of the director-general of the Indian Council of Medical Research (ICMR), N K Ganguly, has been set up, which is expected to come up with a detailed list of laboratories storing the virus. But this may well take a good two years. ICMR has already written letters to all ministries to give a list of laboratories under them.
Experts explain that this is a formidable task: India has a large number of private laboratories. One, enumerating these laboratories may not be easy as there is no legal or other requirement for their registration and second, even collection of reports from them would be quite formidable. A district list would need to be prepared with help from the district health officers.
Vet Microbiol. 2004 Sep 8;102(3-4):131-40. Related Articles, Links
Comparison of the sensitivity of in vitro and in vivo tests for detection of
the presence of a bovine viral diarrhoea virus type 1 strain.
Antonis AF, Bouma A, Bree Jd J, De Jong MC.
Division of Infectious Diseases and Food Chain Quality, Animal Sciences
Group, Wageningen University and Research Centre (WUR), P.O. Box 65, Lelystad
AB8200, The Netherlands.
Veterinary vaccines are usually tested for the absence of contaminants. However, the quality control does not always imply that vaccines are not contaminated as, for example, illustrated by the bovine herpes virus 1 (BHV1) vaccine used in The Netherlands in 1999 that contained a small amount of bovine viral diarrhoea virus (BVDV1). Thousands of cows were vaccinated with BHV1 vaccine batches, and the question arose as to whether these small amounts of BVDV1, most likely not detected with in vitro tests, could have infected cattle. More in general, the question was whether the outcome of the in vitro tests, i.e. the in vitro infectivity, was indicative for the infectivity for cattle, i.e. the in vivo infectivity. We therefore carried out in vitro experiments to determine the sensitivity of a BVDV1 isolation assay. In addition, we performed two animal experiments, in which we estimated the lowest dose needed to infect calves with BVDV1. We extrapolated the experimental in vitro and in vivo results from a tissue culture infectious dose (TCID(50)) to a cattle infectious dose (CID(50)). We observed a partial response in the calves inoculated with this dose: four out of six calves turned out to be infected. In the tissue culture test, all 20 samples tested negative. The response in vivo, however, was not significantly higher than the in vitro response, which implies that no difference in susceptibility was observed between the animal test and the tissue culture test. Based on the results in our experiments, some cattle may have been infected with BVDV1 after the application of the contaminated BHV1 vaccine during the vaccination campaign. The question remains that how many cattle received contaminated vaccine, and became infected with BVDV1.
PMID: 15327789 [PubMed - in process]
http://www.nepalnews.com.np/archive/2004/sep/arc_sep04_43.htm#3
Three dozen children fall sick following immunization against measles
Three dozen children in the Dhungesanghu VDC of Taplejung district, who were vaccinated against measles in the recently held immunization campaign, have fallen sick. The state of ten of them is reported to be critical. Some of the sick children have developed cough, fever, chest pain, shivering, loss of appetite and wounds in the mouth while some have even vomited blood, Nepal Samacharpatra reported today, quoting parents of the sick children. The children were vaccinated against the deadly disease during the firstphase of the immunization campaign held on September 21. “I don’t know what happened. But my daughter has become ill after she was administered the vaccine,” the daily quoted one Ambika Nepali, a local guardian, as saying.
Following the news, the Taplejung district hospital had dispatched a medical team to the VDC, which lies some 12 kilometers away from the district headquarters. Though the team distributed antipyretic drugs like Cetamol and Ketomox in the village, more children fell sick, the daily said quoting another local named Durga Maskey.
nepalnews.com amt Sept 27 04
http://kyw.com/Local%20News/local_story_302163549.html
Oct 28, 2004 6:08 pm US/Eastern
PHILADELPHIA (KYW) A lab worker from Jefferson Hospital has been contaminated with a virus that is used in the smallpox vaccine. Medical Reporter Stephanie Stahl has learned that the female worker was treated at Wills Eye Hospital for an eye lesion. She was then sent back to Jefferson Hospital where she remains under quarantine. The Philadelphia Health Department confirms that the incident is a case of Vaccinia and it has in fact been isolated.
Stahl reports that Vaccinia is the virus that’s used in the creation of the smallpox vaccine. The virus is related to smallpox but is a much milder and less deadly form that is frequently used for research purposes. In addition, the virus is sometimes used in genetic research to help create other vaccines. Vaccinia can in no way cause smallpox and Stahl says that there is no public health concern as the situation is contained. However, the virus is contagious with close contact. Officials from Jefferson and Wills Eye Hospitals refuse to comment about the situation, citing it is a violation of patient confidentiality. At this point, the condition of the worker is unclear.
http://www.mlive.com/news/fljournal/index.ssf?/base/news-24/1099932606257390.xml
Woman says overvaccination caused son's autism
FLINT
THE FLINT JOURNAL FIRST EDITION
Monday, November 08, 2004
By Shantell M. Kirkendoll
skirkendoll@flintjournal.com • 810.766.6366
QUICK TAKE
Too much of a good thing?
* Children can end up with too many vaccinations or be denied entry to school if state records are not updated. Parents should be sure their doctor or clinic reports vaccines to the Michigan Childhood Vaccination Registry within seven days of a child's appointment, and keep a paper record of vaccinations as backup.
FLINT - Syrus Davis' mother wanted him protected from measles, mumps, hepatitis and polio, but at age 6 he's had nearly twice as many immunizations needed to protect him from those diseases. It's been too much of a good thing, said Yashica Neal of Flint. She said she believes the childhood vaccines poisoned Syrus, who was recently diagnosed with autism.
She blames her pediatrician's office for failing to report vaccinations on time to a state registry designed to prevent overvaccination. Because of the snafu, Syrus received more shots than he needed, she said. "He changed after all those shots," Neal said. "He's gloomy and lost." While most children need only four doses of the polio vaccine, Syrus has had six doses, according to state records. By 1999, he had five doses of Hepatitis B vaccine, when he only needed two. It's been only recently that Syrus started talking. Late speech is a classic sign of autism in which children are socially withdrawn and lack language skills.
Like many parents and autism groups, Neal contends the mercury in vaccines is linked with autism. That contention is hard to dismiss because of a marked increase in autism in the past decade that experts cannot explain. Research has been little help. The Institute of Medicine issued a report in May concluding no causal link between vaccines and autism, but a month later, a study published online by the journal Molecular Psychiatry showed it did cause autism-like symptoms in some newborn mice.
But "no one has studied what happens when children are overvaccinated," said Neal, 29. "It couldn't have been good for him." Syrus was born in 1997, the same year the Michigan Childhood Immunization Registry went into effect. All health care providers who give vaccines are required by law to report every vaccine they give a child.
It's supposed to help parents keep track of vaccines administered at multiple places such as a clinic or doctors' offices. MCIR is considered more reliable than paper records, which can be lost or destroyed. "But when you look up in the system, the shots from the pediatrician are not there," she said. "I think that's what's been going on." Neal took care of Syrus' shots with a pediatrician her family had used for years, but she claims his office reported shots weeks after appointments or not at all.
After Syrus was born, Genesys Home Health and Hospice's Maternal Infant Support Service came to Neal's home to check on his well-being and hers and, based on the MCIR records, gave Syrus vaccinations. A Genesys spokeswoman said Friday that nurses use MCIR as the "gatekeeper" for children's records, and follows federal guidelines for when children need vaccines.
"I remember the day (the nurse) came to the house," Neal said. "He'd already had the shot last month. I knew that, but she said the state record showed he hadn't. Who was I to deny medical help for my son? I listened to her." Most children will have five doses of vaccine to prevent diphtheria, tetanus and pertussis, but Syrus has had seven doses, according to his MCIR record.
Parents may also feel strong-armed into getting vaccines because children cannot attend school or preschool unless records show they have all their shots. Neal became alarmed about the shots when Syrus' preschool told her in June he wouldn't be able to attend school because state records showed he needed another measles-mumps-rubella shot. Syrus' doctor's records show he had the
shot in October 2001.
Syrus' pediatrician did not return calls seeking comment. Neal has contacted an attorney who may file a lawsuit against him, she said. "Making sure the data is there is something providers have to buy into," said Wendy Nye, director of the local MCIR region. "Most recognize it as a wonderful tool for tracking vaccinations." Compliance may be affected by an office's volume of vaccinations to be entered or turnover of staff trained to do it, she said.
She said 95 percent of Genesee County doctors are connected to the registry and vaccinations must be on the registry within seven days. "Occasionally parents dispute MCIR, and that's usually when a child has more shots on their (immunization card) from the doctor's office than the registry," she said.
Generally, schools will accept a doctor's note or record as proof of a vaccination, even if MCIR shows otherwise, Nye said. Health officials say few vaccines given U.S. children today contain the preservative thimerosal, which is used to store multiple doses of some vaccines such as for hepatitis B. Thimerosal contains mercury.
The U.S. Centers for Disease Control and Prevention and the American Academy of Pediatrics in 1999 recommended a switch to mercury-free vaccines. Most of Syrus' shots were given more than two years before the switch. "The amount of shots Syrus has gotten is unacceptable," said LaQuantus Cardwell, 30, his aunt and an office manager in a pediatrician's office. "No one knows whether it was intentional, but we do know Syrus will be this way for the rest of his life."
© 2004 Flint Journal.
http://www.sun-sentinel.com/news/local/florida/sfl-1020flushots,0,3196621.story
?coll=sfla-news-florida
"In Central Florida, the vaccine mix-up was discovered Monday, when Get Healthy Florida received about 6,000 doses of influenza vaccines labeled "Shire." "Shire Pharmaceuticals Group is a British company that sold its vaccine division in September to a Canadian company, ID Biomedical."
http://news.bbc.co.uk/1/hi/wales/mid/4017559.stm
Vaccine scare boy 'improving'
A teenager is "improving" in intensive care after a severe allergic reaction to a vaccination at a school in Aberystwyth. Dominic Hamer, 13, is off a ventilator and talking. It is hoped he will be transferred to a paediatric ward at Bronglais Hospital later.
Ten other pupils from Ysgol Gyfun Penweddig were also treated after "adverse effects" to the injections. Two other pupils will be discharged on Wednesday, said the hospital. Nearly 120 pupils at the comprehensive school were being injected with the BCG vaccine, which combats tuberculosis.
A Ceredigion NHS Trust spokesman said it was investigating, and had stopped the batch of vaccine being used. Dominic was said to have had an anaphylactic shock, which is defined as a very sudden serious physical reaction caused by an allergy. Three fellow pupils were kept in overnight for observation, but the rest were discharged.
Bronglais Hospital chief executive Alison Williams said Dominic had improved overnight. She said on Wednesday that medical staff "hope to move him to a paediatric ward later today and send him home at the end of the week". The trust has said it is working closely with the school to ensure that
pupils and parents receive any information or support they require. Individual advisory letters have also been issued to pupils. Brian Thomas, head of corporate services at the trust, confirmed the batch of vaccine had been withdrawn.
Intensive care
A statement from the trust said the batch of vaccines used at the school had also been used on Monday without any adverse effects. A team of four doctors and three nurses had been conducting the vaccinations at Penweddig Comprehensive.
The statement said: "At 12.30pm, nine children presented at the accident and emergency department of Bronglais General Hospital following a suspected adverse reaction to a BCG immunisation. "Another two pupils attended the department in mid afternoon." "Following assessment, 10 of the pupils were admitted for observations and one pupil admitted to the intensive care unit."
Reaction
Dr Mike Simmonds, the Welsh Assembly Government's Senior Medical Officer, said youngsters who are receiving the BCG vaccination are first given a "star" injection in their forearm. "If you don't get a reaction to that you then go on to get the actual vaccine which is given actually within the layers of the skin".
Dr Simmonds told Radio Wales that reactions to vaccinations to children were "incredibly rare". Only 80 cases had been identified in a three-year period among the millions of vaccinations administered across the UK. "All these diseases that we are protecting against have had a horrible track record," he said. "TB is a long-term illness. In the past people were in hospital for weeks and weeks if they survived that illness. "Measles just kills and can kill very rapidly, and is still one of the biggest killers across the world".
http://www.newindpress.com/Newsitems.asp?ID=IEA20041122141524&
Title=Southern+News+-+Andhra+Pradesh&Topic=0
Infants die; reaction to polio drops feared
Tuesday November 23 2004 00:36 IST
KHAMMAM: In a shocking incident, three infants died, after allegedly developing reaction to polio drops administered on Sunday in Penuballi mandal of this district.
According to information reaching here, all the three infants, aged under two months, suffered from shivering after being given polio drops. One kid died on Sunday and two others succumbed on Monday. Unconfirmed reports said that another infant died in the mandal due to a similar reaction.
The victims were identified as R Venkat Narsaiah of Bramhalakunta, Pramila (Karaigudem) and another baby girl who is daughter of one K Narasimham Kumar in Upplachalaka village. The parents alleged that the deaths were a result of complications arising out of polio drops and that the children were normal and healthy before.
Dr Gopala Krishna and Dr Ranga Prasad from Hyderabad, who are a part of the rapid response team of WHO, visited the district to obtain a report on the deaths.
When contacted, district medical and health officer Dr R Krisha Bramham maintained that the deaths were unrelated to polio drops. ``It is a coincidence that the deaths happened just a day after polio drops were given,'' he said.
Polio dropped from list of suspects
http://timesofindia.indiatimes.com/articleshow/935121.cms
November 25, 2004
HYDERABAD: Two more babies who had been given polio drops last week died at Penuballi and Aswaraopet mandals in Khammam district on Tuesday, but experts and government officials took pains to explain that the vaccine could not possibly have caused the deaths. These two deaths and one more in Anantapur on Tuesday took the number of post-inoculation mortalities to seven.
A postmortem on the exhumed bodies of four children who had died over the weekend showed that they had succumbed to neonatal complications. Health officials said none of the seven deaths could be attributed to the oral polio vaccine drops. Nevertheless, the incident is being "critically viewed" and samples of the vaccine given to the children are being sent to the Central Drug Research Institute at Kasauli.
Family welfare officials said the children, most of them months old, either had a medical history or died of reasons not linked to the polio drops. It was a coincidence that the deaths have occurred after inoculation.
Dr R Gopalakrishna Rao, joint director of the family welfare department, said two of the Khammam deaths were due to asphyxia (suffocation) caused by vomit— "not caused by the polio drops"—and one was due to intestinal obstruction. Some of the deaths could have occurred due to some substances being administered to the babies by the mothers: one had received oil drops in her nose and one was given a sugar syrup.
One of the seven children who died was a premature baby who had developed respiratory problems after swallowing amniotic fluid during birthr. A five-year-old girl who died in Kurnool was suffering from fits, Dr Rao said.
"The deaths are a sheer coincidence. We are 100 per cent sure that the deaths have nothing to do with polio drops," Dr Rao said.
Principal secretary (medical and health) I V Subba Rao said the post mortem reports proved that the deaths were due to the vaccine.
http://elmundosalud.elmundo.es>
The Sanitation Ministry is retaining a batch of yellow fever vaccine
--------------------------------------------------------------------
The Sanitation Ministry reported today [26 Oct 2004] that they are retaining a batch of yellow fever vaccine as a "preventive measure," after a 26-year-old woman from Onuba died from yellow fever stemming from a post-vaccination reaction. The woman was admitted last Thursday [21 Oct 2004] to Juan Ramon Jimenez Hospital in Huelva because of fever and multi-organ failure, having had, in addition to fever, malaise, vomiting, and diarrhea during the previous days.
According to a press release from the hospital, since she was about to travel abroad, the patient was also vaccinated for diphtheria and tetanus on 14 Oct 2004 at the External Sanitation Services in Huelva, an office of the Sanitary and Consumer Affairs Ministry. The patient was directly admitted to intensive care, where she was diagnosed with yellow fever associated with a post-vaccine reaction that rapidly progressed and could not be successfully controlled by the medical team, and she died last Friday [22 Oct 2004] at 5:30 AM.
Sources from the Sanitation Ministry reported that they are waiting for new information from the Andalucia Council regarding this case. They also emphasized that people "should still have their vaccines if they have to travel abroad," since the benefits of immunization outweigh the risks.
No past history of such an event in Spain
-----------------------------------------
The same sources gave assurances that the performance of the Huelva Vaccination Center was "perfect" with respect to the protocol, and they have tried to avoid generating unnecessary alarm, insisting that "the batch has been localized."
The Sanitation Ministry commented that "since 1985, there have been only 25 communications reporting mild symptoms considered as vaccine reactions, but not a single case similar to that reported in Huelva." Accordingly, the Sanitation Ministry indicated that the frequency of cases similar to the one reported today [26 Oct 2004] for the age group between 25 and 44 years is less than zero percent per 100 000 inhabitants. The Ministry is waiting for information about the case from the
Andalucia Council; consequently, the vaccine batch retention "is only a preventive measure issued by the Sanitation Ministry," they pointed out.
The Management at Juan Ramon Jimenez Hospital confirmed that samples were sent to the Majahonda National Center for Microbiology and Virology soon after the victim was admitted to the hospital for biologic confirmation of the case.
Yellow fever vaccine, which is manufactured using attenuated viruses, is not included in the vaccine calendar of the Andalucia Council, since it is a disease absolutely absent in Andalucia and in Europe. The batch immobilization is a measure recommended by the External Sanitation Department, which belongs to the Ministry of Sanitation and Consumer Affairs, to be complied with only in case of traveling to countries where the disease is still present: i.e., the majority of sub-Saharan countries and tropical countries in the Americas.
--
Pablo Nart
<p.nart@ntlworld.com>
[Brazil, USA, and Australia have also reported such cases (see refs. below). This case does not seem to correspond to problems with the vaccine (contaminated batches), or with manufacturing problems, but is a consequence of a special interaction between the virus in the vaccine and the host; since, in rare cases, there might be an inability by the host to generate an adequate immune response to the virus. - Mod.LJS]
[see also:
2002
----
Yellow fever vaccine-associated deaths - MMWR report 20021108.5756
2001
----
Yellow fever vaccine-associated deaths reported 20010715.1367
Yellow fever vaccine-associated deaths (02) 20010727.1467
Yellow fever vaccine-associated deaths (05) 20011201.2925
2000
----
Yellow fever - Brazil: vaccine deaths 20000326.0436
Yellow fever - Brazil: vaccine deaths (02) 20000328.0452]
http://www.sunnetwork.org/news/regional/andhra/andhra.asp?id=10860
Sun Network, India
Four babies `die after polio vaccination'
Khammam, Nov 23 - Four babies, all hardly two-months-old, died in Penuballi and Sathupalli mandals within 24 hours of the pulse polio immunisation programme. Parents of the babies blamed it on the side effects of the vaccine. Members of the rapid response team deputed by the World Health Organisation and medical teams from Hyderabad were on their way to the villages.
According to reports, Laksmi, a two-month-old baby, died in Uppalachalaka village today. She was administered the pulse polio vaccine around 9.30 a.m. A two-month-old baby, Rajaneni Venkatanarsaiah, died in Brahmalakunta village in the day, after being administered the pulse polio drops around 10 a.m on Sunday.
Some 145 children below two years were covered under the pulse polio vaccination programme in the village. The medical and health authorities said that all others who were given the vaccine were safe.
http://timesofindia.indiatimes.com/articleshow/935121.cms
The Times of India
Polio dropped from list of suspects
THURSDAY, NOVEMBER 25, 2004 12:43:44 AM ]
HYDERABAD: Two more babies who had been given polio drops last week died at Penuballi and Aswaraopet mandals in Khammam district on Tuesday, but experts and government officials took pains to explain that the vaccine could not possibly have caused the deaths. These two deaths and one more in Anantapur on Tuesday took the number of post-inoculation mortalities to seven.
A postmortem on the exhumed bodies of four children who had died over the weekend showed that they had succumbed to neonatal complications. Health officials said none of the seven deaths could be attributed to the oral polio vaccine drops. Nevertheless, the incident is being "critically viewed" and samples of the vaccine given to the children are being sent to the Central Drug Research Institute at Kasauli.
Family welfare officials said the children, most of them months old, either had a medical history or died of reasons not linked to the polio drops. It was a coincidence that the deaths have occurred after inoculation.
Dr R Gopalakrishna Rao, joint director of the family welfare department, said two of the Khammam deaths were due to asphyxia (suffocation) caused by vomit- "not caused by the polio drops"-and one was due to intestinal obstruction. Some of the deaths could have occurred due to some substances being administered to the babies by the mothers: one had received oil drops in her nose and one was given a sugar syrup.
One of the seven children who died was a premature baby who had developed respiratory problems after swallowing amniotic fluid during birth. A five-year-old girl who died in Kurnool was suffering from fits, Dr Rao said. "The deaths are a sheer coincidence. We are 100 per cent sure that the deaths have nothing to do with polio drops," Dr Rao said. Principal secretary (medical and health) I V Subba Rao said the post mortem reports proved that the deaths were due to the vaccine.
http://abcnews.go.com/Health/wireStory?id=232833
Doctor Accused of Using Old Flu Vaccine
Washington State Accuses Physician of Giving Outdated Flu Vaccine to 55 Patients
BELLINGHAM, Wash. Nov 6, 2004 — The state's medical quality board has accused a physician of giving outdated flu vaccine to 55 patients. Dr. Gary McCallum used a batch of last year's vaccine, according to the state's Medical Quality Assurance Commission, which licenses physicians and other medical professionals. The board charged him on Friday with unprofessional conduct, specifically fraud and misrepresentation. McCallum told patients the vaccine, purchased last year in Canada, was good for the current flu season even though its expiration date was in July, state Health Department spokesman Tim Church said. "The people that received the vaccine aren't at any increased risk of side effects, but they may think they've been immunized when they … have not been immunized," said Regina Delahunt, director of the Whatcom County Health Department.
Flu vaccine is custom-made each year for the types of virus expected during the coming flu season. About half of the nation's supply for this winter was eliminated because of contamination at one drug company's laboratories in Britain.
McCallum, of Barkley Village Family Medical Clinic, said all affected patients were getting new shots and that he could not comment further. McCallum administered the vaccine between Oct. 15 and Tuesday, charging $20 for adults and $10 for children. Health Department investigators visited McCallum's practice Wednesday after receiving a tip, Church said.
http://www.newsday.com/news/nationworld/wire/sns-ap-injection-scare,0,970987,print.story?coll=sns-ap-nationworld-headlines
Nurse in Flu-Shot Scare Claims Innocence By JOSHUA FREED
Associated Press Writer
December 13, 2004, 5:07 AM EST
MINNEAPOLIS -- The woman offering flu shots for $20 in the commons area at Augsburg college seemed plausible enough -- green scrubs, white lab coat, stethoscope -- that some three dozen people willingly paid their money, rolled up their sleeves and let her plunge the needle in.
But no one had scheduled a flu clinic. No one knew who the woman was. And no one could be sure what was in those syringes. She turned out to be 33-year-old Michelle Torgerson, a freelancing nurse who claims she was selling leftover vaccine to raise money for a fund-raiser at her daughter's school. And those shots were certainly nothing sinister, if not just plain old flu shots, lab tests have found.
But in an age when bioterrorism experts worry about sophisticated attacks, the case shows how anyone with a syringe and a reasonable looking get-up can have potential victims lining up -- even paying -- to be injected with who-knows-what.
"Imagine if they hadn't been able to track her down, and they had no idea, and somebody had just come onto a college campus injecting people with something," said Dr. Michael Osterholm, a bioterrorism expert with the National Center for Food Protection and Defense at the University of Minnesota.
Osterholm said it's not realistic to expect patients to challenge the credentials of every nurse who administers a flu shot -- but he said the school should have. "I think this really is at the center of the discussion about what does it mean to recognize and report suspicious behavior," he said. On Dec. 2, a staffer confronted Torgerson, asking who she was and who her supervisor was, according to Augsburg security director John Pack. Pack said he and another security worker headed toward the commons to investigate. "At that point I wasn't thinking whether or not it was malicious or a mixup," Pack said. "I was thinking about the scope -- I wondered how many people received shots."
Torgerson, of suburban Albertville, had left the campus promising the staffer she would provide the name of a supervisor, Pack said. But the security director said she never did, further raising suspicions.
Torgerson gave a different account Sunday, saying she did speak briefly to an administrator named "Diane," but that she wasn't challenged. She said she left after the noon hour, as she had planned, and did not "abruptly" leave, as officials have said. Pack called police, and then set about trying to find out how many people had been injected. He sent an e-mail alert to everyone on campus, and printed red fliers asking anyone who had gotten the shots to come forward. Teachers read the alert in class the next day.
Police arrested Torgerson the day after the injections were discovered, near her ex-husband's home in Belgrade, 90 miles away from the college. Torgerson, a licensed practical nurse who had given immunizations as part of a legitimate clinic at Augsburg last month, insisted Sunday she did nothing wrong. She said she believed she did have permission from an Augsburg administrator in charge of the student center to give the flu shots on her most recent visits to the school.
Torgerson has not been charged.
But some students are going in for HIV tests anyway, and about 100 attended a meeting in the school's chapel last week where police and health workers answered questions. Augsburg president William Frame apologized. Some students were mollified, while others remained angry. According to the Minnesota Department of Health, test results that came back Friday from the Food and Drug Administration showed the vials police took from Torgerson contained real flu vaccine, but the vaccine in some vials had been watered down with saline solution. On Sunday, Torgerson emphatically denied diluting the vaccine, and her lawyer disputed the accuracy of the test results, saying they'll bring in their own experts.
Torgerson, who had worked at several immunization clinics, has said her employer told her to dispose of the leftover vaccine. Employer Maxim Healthcare Services of Columbia, Md., said Torgerson was told to return all her unused vaccine by Nov. 12, the day after her last authorized flu clinic. Torgerson disputed that too, saying her supervisor told her was trying to line up other flu shot clinics.
Some bioterrorism experts said it's tough to guard against a theoretical threat like someone impersonating a nurse and injecting victims with something toxic. "Society operates at a certain level of trust, and I think it's very difficult to really police ... any sort of activity like this very extensively," said Dr. D.A. Henderson, a senior adviser at the Center for Biosecurity at University of Pittsburgh Medical Center.
"There are reasonable fears that we have, and there are a million potential things that we could imagine someone might do if they really were nasty about it," he said. Augsburg now requires outside vendors to display a permit. But Pack said Torgerson seemed to be trying to answer the staff person's questions, even promising to call back with the name of a supervisor.
"She certainly acted like she belonged," he said.
Associated Press writer Steve Karnowski contributed to this story.
Copyright © 2004, The Associated Press
http://www.woai.com/news/local/story.aspx?content_id=CFCBDB02-665F-4844-BEB1-73A84C1C0F51
Vaccination Double Dose
A local mom made sure her daughter was vaccinated against deadly diseases, but the state of Texas has now forced her to double dose her child. Now she's scared these repeat shots may be dangerous.
“Every time she comes down... getting sick, I'm going to worry,” Deborah Keller says of her daughter. “If she's slow at learning something at school, I'm going to worry.” Deborah's worried because her 4-year-old daughter was forced to repeat her polio, diphtheria and tetanus shots because of a new state law. The law went into effect after school nurses said they wanted immunization schedules to be based on grade level, not birthdates.
Her daughter's birth date is June 22nd. She received her shots June 11th. That brought a phone call from the school. “They called me last week - over a year after she's had her shots - telling me that in April of this year, the law has changed and she has to have those shots within four days of her birthday,” Deborah explained. “There are some that are getting caught in the crossfire.”
Deborah's daughter is one of them. She's in kindergarten
“The state has allowed first through 12th graders to be grandfathered,” she said. She says she never heard about the new law. “We had done some interviews for news and done some promotions in April and May,” countered Linda Lopez, a registered nurse at the San Antonio Health District. But no official notification.
“I don't know if the State made any announcement except on their web page,” said Lopez. But now it's the law; whether it makes sense or not. “The shots are good for ten years, whether she gets them eleven days before her birthday or four days before her birthday.” Health officials say the second round of vaccinations should not be harmful to Deborah's daughter. I
January 24, 2005 Exposure at Germ Lab Reignites a Public Health Debate By SCOTT SHANE
ast year, while working on a vaccine to protect against bioterrorist attacks, three laboratory workers at Boston University were exposed to the bacteria that cause a rare disease called tularemia, or rabbit fever.
The workers recovered, though two of them had to be hospitalized. But the prognosis is less certain for the university's ambitious plan to build a high-security biodefense laboratory, part of a national boom in germ defense research touched off by the Sept. 11 attacks and the anthrax letters of 2001.
The tularemia episode, acknowledged by university officials only after inquiries last week from the news media, has outraged opponents of the proposed $178 million laboratory and reignited a national debate over whether the rapid expansion in work with dangerous pathogens is adequately regulated and scientifically justified.
The Boston case follows other mishaps in germ research, including the accidental shipment of virulent live anthrax from Maryland to California last March, and an investigation that revealed multiple spills of anthrax bacteria in the Army's biodefense laboratory. Such incidents have led some scientists to ask whether the growing number of germ laboratories - financed from the $14.5 billion in federal money spent on civilian biodefense since 2001 - may pose a menace to public health comparable to the still uncertain threat from bioterrorism.
Dr. David Ozonoff, a professor of environmental health at the Boston University School of Public Health who originally supported the new laboratory but now opposes it, argues that biodefense spending has shifted money away from "bread-and-butter public health concerns." Given the diversion of resources and the potential for germs to leak or be diverted, he said, "I believe the lab will make us less safe."
Dr. Mark S. Klempner, associate provost for research at Boston University's medical school, says the proposed laboratory, to be designated a National Biocontainment Laboratory along with one to be built in Galveston, Tex., will pose no public hazard. To be designated Biosafety Level 4 - the highest level of security - it will develop drugs and vaccines to protect not only against bioterror agents but also such natural emerging diseases as SARS and West Nile virus, he said.
"The nation needs this lab," Dr. Klempner said.
Such disparate views among scientists reflect deep uncertainty about the scale and imminence of the bioterror threat. Some experts believe an attack that could kill tens of thousands of people is plausible today. Others argue that the known terrorist groups have little sophistication about biological weapons. Instead, these critics say, the biodefense expansion has been fueled by a scramble for federal money.
Currently there are four Biosafety Level 4 laboratories nationwide, with six more planned; 50 laboratories operate at Biosafety Level 3, sufficient to work with anthrax, and 19 more are planned at universities and government institutions, according to the Sunshine Project, a Texas group that is tracking the growth.
In the only major bioterrorist attack in American history - the anthrax-laced letters mailed to news media figures and two senators in fall 2001, killing five people - F.B.I. investigators have focused chiefly on the theory that the anthrax originated not with outside terrorists but within an American biodefense program.
By the same token, the critics say, the tularemia that sickened the workers in Boston would not have existed if not for bioterror research. Dr. Richard H. Ebright, a molecular biologist at Rutgers University, said the disease "has zero public-health importance." Only about 130 cases a year are reported in the United States.
The flood of biodefense financing has drawn hundreds of inexperienced researchers into work with hazardous organisms, Dr. Ebright said. The Boston accident, he added, "shows gross, basic incompetence and raises real questions about the competence of that institution to run a biosafety Level 4 lab."
Boston University officials concede that the tularemia vaccine researchers did not follow proper safety procedures and have removed the principal investigator, Dr. Peter A. Rice, from his post as chief of infectious diseases. Dr. Rice was to have had a role training workers for the proposed high-security laboratory.
University officials say the tularemia vaccine researchers thought they were working with a harmless "vaccine strain" of the Francisella tularensis bacterium. But for reasons unexplained, the sample was mixed with a virulent strain.
When two laboratory workers developed flulike symptoms in May, no one tied the illnesses to the research, said Dr. Thomas J. Moore, acting provost of the university's medical campus. Only after a third worker was hospitalized for several days in September did Dr. Rice first pursue the possibility of tularemia.
Dr. Moore said the tularemia diagnosis was confirmed on Oct. 29 but not reported to state health authorities until Nov. 9, a delay he said he could not explain. But he defended the decision not to tell the public.
"I feel comfortable about the decision not to make a public announcement because there wasn't a public risk, since tularemia can't be passed from person to person," Dr. Moore said.
Opponents of the proposed laboratory see things differently. Douglas H. Wilkins, a Boston lawyer who filed a lawsuit on Jan. 12 challenging the laboratory plan on behalf of 10 neighborhood residents, noted that the university's environmental impact statement claims the medical center has "not had any laboratory-acquired infections."
Tomas Aguilar of Alternatives for Community and Environment, a group opposed to the laboratory, said: "Three infections in a five-month period - and this is all going on when Boston University is saying how safe this dangerous laboratory is going to be. A lot of people are saying, 'It's even worse than we thought.' "
Similar safety questions have been raised by two other incidents. In 2002, the discovery of lethal anthrax outside a high-security laboratory at the military's premier biodefense laboratory, the Army Medical Research Institute of Infectious Diseases at Fort Detrick in Maryland, led to sampling throughout the institute. Investigators found three different strains of anthrax bacteria outside the sealed-off laboratories, indicating at least that many leaks, according to an Army report.
Then, last spring, Southern Research Institute, a contractor in Frederick, Md., shipped anthrax bacteria to an Oakland, Calif., hospital after immersing it in hot water to kill the germs. When mice injected with the supposedly harmless bacteria for a vaccine experiment quickly died, researchers realized the bacteria were still lethal.
Those incidents produced no human illnesses. But Dr. Ebright said some current research poses a much higher risk, notably the work by several groups that are trying to reconstruct the 1918 influenza virus, which killed more than 20 million people.
"This work is being done in the absence of any real oversight," he said.
One scientist who supports the increase in biodefense spending, Dr. Tara O'Toole, does not dismiss the safety issues. In fact, she said, the biodefense expansion has focused attention on long-neglected biosafety rules. But she believes the danger of bioterrorism is so great that the billions of dollars being spent on protections may not be enough.
"I think bioterrorism is the biggest national security threat of the 21st century," said Dr. O'Toole, director of the Center for Biosecurity of the University of Pittsburgh Medical Center. "So I want a robust biodefense research and development program."
Dr. O'Toole recently helped organize a bioterror exercise, called Atlantic Storm, in which terrorists attack with smallpox in the United States and four foreign countries, killing more than 87,000 people. Such a potential toll puts the risk of laboratory accidents in a different perspective.
But is that situation realistic, when nothing remotely approaching such an attack has ever occurred?
"After 9/11, I don't think anyone would say that just because an attack hasn't happened, it can't happen," Dr. O'Toole said. "I keep trying to talk myself out of this. But it just keeps getting scarier and scarier."
Parents still suspect BCG vaccine after many children fell ill at school Jan 26 2005
http://icwales.icnetwork.co.uk/0100news/0200wales/tm_objectid=
15116700%26method=full%26siteid=50082%26headline=%2dparents
%2dstill%2dsuspect%2dbcg%2dvaccine%2dafter%2dmany%2dchildren%2dfell
%2dill%2dat%2dschool-name_page.html
Gareth Morgan, Western Mail
PARENTS of a schoolboy who was put into intensive care after receiving the routine BCG vaccination, say they are not satisfied with claims that the vaccine was not faulty. Dominic Hamer, 13, suffered anaphylactic shock - a severe allergic reaction - after the anti- tuberculosis jab was administered at Ysgol Gyfun Penweddig in Aberystwyth last November. There was speculation at the time of the incident that other unwell pupils merely panicked when Dominic fell genuinely ill.
The local NHS trust has now admitted the incident was more than just "hysteria". But in a letter to parents it also insisted there was nothing wrong with the storage, transport or administration of the BCG vaccine.
Dominic's parents say they do not know where to turn, after the letter insisted the vaccine was definitely safe. Yet it also led to 12 other school pupils being taken to hospital. Paul and Maria Hamer said, "We are not satisfied, but we do not know where to go to get any more answers. "We are not interested in compensation, we just want to know what happened and why." Ceredigion and Mid Wales NHS Trust said its findings are correct and have been confirmed by the Department of Health.
It did note that some of the other pupils' problems were reported before Dominic's attack, making an "anxiety reaction" in response to what happened to their friend unlikely. But it concluded that other pupils suffering symptoms like dizziness, nausea and breathing difficulties were experiencing side effects that were "severe, but normal". Last night Mrs Hamer, a nurse, said, "It seems incredible that so many children were affected so badly, all at once.
"At least they admit now that it was more than just a mass panic attack. "But if it wasn't a panic attack, we certainly do not know what did cause the problems, or how we will find out. "These are supposed to be the experts, but they have not told us much and we do not know who else to ask." His father Paul, a garage mechanic, added, "The trust admits that there wasn't mass hysteria by students and that the problems the students had were normal. "But it's a bit alarming that so many people were affected by BCG at the same time at the same place.
"There's been an investigation and that's it - until something happens again." But Mr Hamer, whose own grandfather died from TB, has always urged parents not to withdraw their children from routine vaccinations. Despite his own son's reaction to the jab, he said that maintaining public immunity to the disease is important. Dominic, from Bow Street,near Aberystwyth, returned to school eight days after his ordeal. He had spent a night in intensive care with breathing difficulties, before being transferred to a general ward. Mrs Hamer said, "He is great now, all the children are fine, which is the most important thing. "It was a short-term effect thankfully and he is now back to playing his rugby again."
Even after his severe reaction to the BCG, the then-Welsh Health Minister Jane Hutt said there were no grounds for suspecting any fault with the vaccine.
Pediatric Dermatology
Volume 22 Issue 2 Page 130 - March 2005
doi:10.1111/j.1525-1470.2005.22208.x
http://www.blackwell-synergy.com/links/doi/10.1111/j.1525-1470.2005.22208.x/abs/
Vaccine-Associated "Wild-Type" Measles
Kelle Liermann Berggren, M.D.*, Michael Tharp, M.D.*, and Kenneth M. Boyer, M.D.
Abstract:
Measles is the most contagious of the childhood exanthems and is the leading cause of vaccine-preventable deaths in children, mostly in developing countries. The prodromal stage, consisting of high fever and the triad of cough, coryza, and conjunctivitis, is followed by a caudal progressing rash over a period of 2 to 3 days. With a worldwide vaccination program in place, mortality and morbidity have decreased substantially. Receipt of the live attenuated vaccine generally causes no or only mild side effects such as a low-grade fever and a subtle rash.
We report a 1-year-old boy who, 10 days after vaccination, developed vaccine measles which was clinically indistinguishable from the natural disease. Vaccine virus was detected by polymerase chain reaction in the patient's nasopharyngeal secretions
Hmmm... looks like the vaccine didn't prevent that vaccine-preventable case!
Flu clinic nurse faces charges
Injections were given in 3 county stores
Mary Lane Gallagher, The Bellingham Herald
http://news.bellinghamherald.com/stories/20050324/TopStories/236471.shtml
Investigators still aren't sure what was in the syringes at flu shot clinics offered at three Lynden and Blaine grocery stores last fall, but prosecutors have charged the nurse who allegedly administered them with practicing medicine without a license.
Whatcom County Prosecutor Dave McEachran charged Nancy Jean Olson with injecting seven people with "a purported flu shot," according to the charging documents. Prosecutors allege Olson injected at least 130 people at grocery stores in Lynden and Blaine in November 2004. Olson, a Canadian, is now in British Columbia, said Detective Lee Beld of the Lynden Police Department. A warrant is out for her arrest, Beld said, and authorities have asked Olson to turn herself in to police in the United States.
Olson was charged with one misdemeanor count and six felony counts of unlicensed practice of medicine. According to charging documents filed Tuesday in Whatcom County Superior Court, Olson held flu shot clinics in November at Cost Cutter and Food Pavilion stores in Lynden and a Cost Cutter store in Blaine. The $30 shots were offered at the height of a nationwide shortage of flu vaccine. Some people who received the shot complained to the store when they didn't get the side effects they expected. Store managers then discovered the nurse administering the shots provided a false company name. Olson later told investigators that she used the name "Pacific Coast Consultants" when working with grocery stores "to make her sound more professional," according to the charging papers.
Investigators determined Olson was the nurse in question after one of her co-workers at Alderwood Convalescent Center in Bellingham spotted her on a television news story about the shots, according to the charging documents. She no longer works in Whatcom County, Beld said. Olson told Lynden police that she had ordered the flu vaccine from the manufacturer Aventis Pasteur, but the company said they had no record of Olson receiving the vaccine or of shipping any to her address.
Then Olson told investigators she had gotten the vaccine from Hoagland's Pharmacy in Bellingham, from a man who had recently died, but according to prosecutors she did not have valid receipts from the pharmacy. "We have gone down the road that she has set before us as to where she says she got the vaccine," Beld said. "We haven't been able to confirm she has ever purchased the vaccine." Nor have investigators concluded what was in the shots, Beld said. Olson gave investigators a half-full vial containing flu vaccine originally sold to Alderwood Convalescent Center, prosecutors said. But tests of empty syringes from a flu shot clinic Olson held at a church in Everson found "a substance not consistent with ... vaccine having been present," according to the charging documents. Some syringes didn't have enough liquid inside to test.
"It's possible that it was saline solution," Beld said, "but we cannot tell for sure." So far, no one has complained of health problems because of the shots, Beld said. "From what the Department of Health has told us, that's a very good sign," he said. Brown and Cole Stores, which owns Cost Cutter and Food Pavilion, sent a letter to customers who said they had received the questionable shots, and offered them legitimate flu vaccine as well as a store gift certificate, said store spokeswoman Sue Cole.
Store managers are also more diligent in checking out non-employees who want to offer health services in stores, she said.
Reach Mary Lane Gallagher at mary.gallagher@bellinghamherald.com or call
715-2285.
50-children fall sick after being vaccinated by polio drops in Jhang
Thursday April 28, 2005 (0502 PST)
http://www.paktribune.com/news/index.php?id=103258
JHANG, April 28 (Online): At least 50 children have been affected by different diseases like cholera, vomiting at the Kashmir Colony, Jhang district after vaccinated by polio drops during polio eradication campaign . The vaccination campaign was launched by the health department Jhang on
April 26, 28 with an objective to eradicate polio from the district. But due to poor quality vaccine 50 children of the Kashmir Colony have been affected by different diseases like Cholera, vomiting after polio teams vaccinated them .
And when parents and school teachers went to the office of Zonal Supervisor Dr. Shahid Bukhari to make a complaint against the grave situation, he become furious and insulted the complainants . After this, the parents rushed towards Executive District Director Officer Health Jhang Dr. Mushtaq Ahmad Shad office carrying a written application but he was not absent from the office . They have called upon government to probe the matter as it is matter of life and death of children who are future of the nation.
End.
Dubious vaccination sickens 120 pupils
www.chinaview.cn 2005-06-25 20:00:23
A six-year-old girl died and at least 121 school children became sick after getting dubious vaccinations in eastern China's Anhui Province, a hospital source said Saturday.
HEFEI, June 25 (Xinhuanet) -- A six-year-old girl died and at least 121 school children became sick after getting dubious vaccinations in eastern China's Anhui Province, a hospital source said Saturday. Without approval from local health and education departments, the epidemic prevention center in Dazhuang Town of Sixian County inoculated bacterin against hepatitis A to more than 2,500 children in 19 primary and middle schools on June 16 and 17.
The girl named Li Wei died in a local hospital Thursday afternoon after contracting fever, diarrhea and twitch. Experts of the provincial sanitation department said at the end of an initial probe that the girl died of serious infection and respiration failure. Legal medical experts from the provincial medical institute dissected her body Friday afternoon but no outcome have been available so far. To console the girl's parents and other close relatives, the relevant local government departments in the county have reached an agreement to pay the girl's family l90,000 yuan (some 10,840 USdollars) in compensation.
Other school kids, aged about seven to 14, are still undergo ingintensive medical care in hospital and 20 of them remain in critical condition, according to doctors. Pan Longgen, the physician-in-charge with the Sixian People's Hospital, said the ailing students' hearts and livers have been harmed to varying extent. But so far, there has been no effective way for treatment, he added.
The bacterin has been sent to the Chinese Ministry of Public Health for testing and the outcome is expected to be available in about 10 days. Enditem
Vaccine incident moral: No room for compromise http://www.chinadaily.com.cn/english/doc/2005-07/02/content_456511.htm
Yan XizaoChina Daily Updated: 2005-07-02 06:58As we get closer to the truth behind the vaccine accident in Sixian County, Anhui Province, the messier things get. Finding the facts appears trickier than we could have imagined. Hundreds of students in a Sixian township have shown unusual symptoms after taking a hepatitis A vaccine the local epidemic prevention agency purchased from a private wholesaler. An affirmative conclusion about the nature and cause of the incident is still pending. There simply were too many variables with potential to cause trouble. We hope the laboratory tests now under way might finally corroborate the judgment of experts that this is purely a rare case of collective hysteria.
However, such an outcome might prove disturbing, because it could discount people's sense of urgency to clear up the mess. All legal requirements about authorization and procedures, as stipulated in the new Regulation on Vaccine Circulation And Inoculation, must be satisfied. Even if violations are not found to be the direct cause of the incident, they could well become the cause of some other incident on another day. There is no room for compromise. Duely followed procedures and authorizations are a matter of life or death in this regard. Beyond that, as the entire picture unfolds, we see it as an imperative to re-evaluate the state of epidemic prevention and control network.
A Beijing News investigation found no clues indicating that a hepatitis A outbreak exists in the region. And some of the students involved had taken a hepatitis vaccine in the recent past and already enjoyed immunity from the disease. In that case, a collective approach to inoculation was unnecessary. An official with the local epidemic prevention station confessed to the newspaper the station had not submitted an application for the inoculation because it knew it would not be approved. But they provided the shots, anyway, because it was a paid service. They bought the vaccine at 4.5 yuan (55 US cents) per shot from a wholesaler, and charged students 25 yuan (US$3) for each injection.
Twenty-five yuan is not a small amount in a place where the farmers' average annual income is a mere 2,000 yuan (US$240). Imposing such an unnecessary financial burden on poor rural households is immoral. The way it was done was illegal. But it was not done without a reason. Epidemic prevention workers wanted money to support themselves. Only four of the six members of the station are covered by the local financial budget. They receive just 150 yuan (US$18) a month.
This is reminiscent of the well-known problems in our discourse of farmers' financial burdens. It offers fresh proof for the popular understanding that overstaffed township administrations constitute an unbearable financial burden on farmers. The outrageous price of the shots - 25 yuan for a 4.5 yuan wholesale cost says it all. Still, we need well-grounded evidence to attribute this malpractice at the township epidemic control station to redundancy. The SARS outbreak two years ago taught us epidemic control was an essential though dange rously weak link in our public health system.
It still is.
The embarrassment of the township epidemic prevention station in Sixian County shows either there are too many people on its payroll, or they are underfinanced. And the way they handled the vaccine inoculations showed they lacked professional training, either in expertise, or in morality.
We need to decide upon reasonable and affordable personnel levels for the epidemic prevention network, so that people can be properly trained and paid to do their jobs. State and local governments share an obligation to provide adequate financial support for epidemic prevention, considering its impacts on people's livelihoods. It is a shame when those who are supposed to serve the public welfare become a threat to it.
http://www.newsday.com/news/local/wire/newjersey/ny-bc-nj--dyfsoffice-death1020oct20,0,1210809,print.story?coll=ny-region-apnewjersey Infant dies after being stricken at DYFS office October 20, 2005, 6:19 PM EDT
NEWARK, N.J. -- An infant died Thursday after being stricken at a downtown office of the state child welfare agency, police said.
The month-old boy, who has been in foster care since birth, had just arrived about noon at an office of the state Division of Youth and Family Services from a doctor's visit. He had received immunizations for hepatitis B and polio at the doctor's, said DYFS spokesman Andy Williams.
"When the aide arrived to bring the baby upstairs, he noticed he was having trouble breathing and there was blood in his nose," Williams said.
The aide brought the child to the office nurse, who began resuscitation efforts while emergency medical workers were called. The first-aid crew took the baby to Saint Michael's Hospital, where he was pronounced dead at 12:46 p.m., Williams said.
"It appears the baby had a reaction to an immunization," Williams said.
An autopsy is to be done to determine the cause of death, police said.
The child, whose name was not released, was placed in foster care immediately after being born Sept. 16, Williams said.
The foster mother had taken the infant to the doctor's. After the visit, a DYFS driver took the boy to the DYFS office, where the infant was scheduled to have a supervised visit with his natural mother, Williams said.
Bereavement counseling is being offered, Williams said.
Copyright 2005 Newsday Inc.
http://www.nytimes.com/2005/10/23/weekinreview/23pollack.html
Lessons From a Plague That Wasn't
By ANDREW POLLACK
Published: October 23, 2005
PUBLIC health experts warn that the world might be close to a repeat of the flu pandemic of 1918, which killed millions. But could it instead be close to a reprise of the 1976 pandemic that never happened?
That is the year President Gerald R. Ford announced a crash program to "inoculate every man, woman and child in the United States" against swine flu. But the virus never became a killer, and vaccinations were halted two months after they began after reports that 500 people who received the shot developed a paralyzing nerve disease and more than 30 of them died.
A vaccination in Watsonville, Calif., in 1976 as part of a crash program to fight swine flu.
If scientists were wrong in 1976, could they be wrong now? Some arguments being made about why a pandemic is looming echo those made three decades ago. But many experts say the situation now is different enough that a false alarm is less likely.
"We just know a lot more about the influenza virus than we did in 1976," said Ira M. Longini Jr., a professor at Emory University who is an expert on epidemics. Still, a lot can be learned both from what did and did not happen back then. The 1976 scare started in February when a handful of soldiers at Fort Dix in New Jersey got sick and one of them died. Scientists determined that the virus was one that infected pigs and was different from the human influenza viruses circulating then. On March 24, barely a month later, President Ford announced the vaccination plan.
One reason for the concern was that scientists thought the 1918 pandemic had been caused by a swine virus and that the Fort Dix outbreak marked its second coming. Furthermore, experts warned that pandemics tended to be cyclical and that another one was about due. Today, thanks to genetic analysis - a technique not available in 1976 - scientists know the 1918 virus was a bird virus that mutated. So now there is concern that the H5N1 avian strain ravaging birds in Asia could in like fashion evolve into a form that can spread easily among people. The avian virus shows some mutations similar to those in the 1918 virus, said Jeffery K. Taubenberger of the Armed Forces Institute of Pathology. And experts are again warning that the world is overdue for a pandemic.
Edwin M. Kilbourne, a professor emeritus at New York Medical College who argued for the vaccination program in 1976, said there was actually less reason to be concerned about a pandemic today. That is because the swine flu virus at Fort Dix clearly passed easily from
person to person, while the current avian flu has not. Many experts disagree, however. In retrospect, they say, the 1976 decision to vaccinate was based on much less solid evidence than is available today.
"Part of the problem was convictions outpacing evidence," said Harvey V. Fineberg, president of the Institute of Medicine, part of the National Academies, and co-author of "The Epidemic That Never Was," a book about the 1976 experience. "I don't think that's
happening today."
Even in 1976 there seemed to be more doubt than there is today about the issue. The World Health Organization and many European nations, for instance, did not see what the fuss was about. Today, they are busily preparing for a pandemic. Doubts grew through the summer of 1976 when the virus was not detected outside Fort Dix. That first death turned out to be the only known one from the virus. Moreover, studies suggested that several hundred people at Fort Dix had been infected but most never got sick.
"That's very different from 120 cases with half the humans dying," said Dr. Fineberg, referring to the approximate toll so far from the Asian bird flu.
Perhaps the biggest mistake in 1976 was giving the vaccine to millions of people rather than just stockpiling it until clearer signs of an epidemic emerged. W. Paul Glezen, a professor at Baylor College of Medicine in Houston, said the prevailing belief was "we should use it because if it started spreading there wouldn't be time." The vaccination program had problems. Production was delayed after one company produced the wrong vaccine. Another delay occurred when manufacturers demanded that Congress protect them from liability. Mass inoculations did not start until October, which might have been
too late had a pandemic begun.
Still, by the time the program was halted in December, only 10 months after the virus was identified, about 150 million doses had been made and 45 million given. There are lessons from 1976 that might be applied to today's preparations. A 2004 draft of a government plan for a pandemic listed some. One is that "making clear what is not known is as important as
stating what is known." Another is that a program should be re-evaluated periodically rather than left on autopilot.
And this time, federal officials said, the vaccine won't be used until there are signs a pandemic is under way. Dr. Fineberg said another lesson is that Congress should provide liability protection for vaccine makers now, rather than waiting until the crisis occurs. He said the wrong lesson to draw from 1976 would be "the superficially obvious one" - that because it didn't happen then it won't happen now and preparations are not necessary.
Still, repeated cries of wolf can make the public blasé.
"There's so much expectation for it to develop into a pandemic that if it does not in the next year or two it's quite possible you would see a backlash like the 1976 experience," said Dr. Taubenberger. "What I fear is that people would make the conclusion, falsely, that influenza is not such an important public health problem."
[PROVE Note: Does anybody really know what is in those vials?]
http://www.chron.com/cs/CDA/ssistory.mpl/front/3422308
Oct. 28, 2005, 8:51AM
1,000 get possibly fake flu vaccine
By ARMANDO VILLAFRANCA
Copyright 2005 Houston Chronicle
More than 1,000 Exxon Mobil employees received flu shots last week with a vaccine now being investigated by the FBI to determine its authenticity. The U.S. Food and Drug Administration is testing samples of the vaccine. The flu shots were given to about 1,000 Exxon Mobil employees and 80 contractors during a Health Fair Oct. 19-20 at the company's Baytown complex of refinery and chemical plants.
Jeanne Miller, a spokeswoman for Exxon Mobil in Baytown, said a state-licensed independent physician's office was contracted to administer the shots. Miller would not identify the office. In the past, she said, a company medical team administered the flu shots to employees at that plant.
She said the FBI notified Exxon Mobil at its corporate offices earlier this week that it was investigating the authenticity of the vaccine. Miller did not know whether the investigation had been ongoing or had stemmed from the use of the vaccine at the Baytown complex.
The FBI in Houston referred all comment to the U.S. Attorney's Office.
In a statement released Thursday, U.S. Attorney Chuck Rosenberg said a preliminary investigation does not show the vaccine to be harmful, but measures were being taken to ensure its safety. "Those known to have received the fake flu vaccine have been notified and steps are being taken to ensure their well being," he stated. "We are also attempting to ascertain whether others might have received a fake vaccine."
If a fake flu vaccine was used, he said, those responsible for its use will be prosecuted for fraud. Miller said all 3,800 employees at the Baytown complex and contractors were notified Wednesday. "We have not received any reports of illness potentially related to the vaccine," she said. She said Exxon Mobil also recommended that employees given the flu shot should be tested for blood-borne pathogens, or germs.
armando.villafranca@chron.com
Vaccine blamed in avian flu outbreak 11/10/2005
asahi.com:Vaccine blamed in avian flu outbreak - ENGLISH The Asahi Shimbun
The agriculture ministry suspects an unauthorized vaccine from Central America caused the avian flu outbreak in Ibaraki Prefecture that could have been spread by farm employees, journalists and the transport of chicken droppings, sources said Wednesday.
The Ministry of Agriculture, Forestry and Fisheries said it was highly unlikely that a migratory bird or imported pet brought the H5N2-type virus to the prefecture in the summer, according to a draft interim report on the issue.
The full text of the report was to be released today.
The first case of avian flu was detected at a chicken farm in Mitsukaido, Ibaraki Prefecture, in June. Chickens at 34 farms mainly in the prefecture were later found infected with the same strain of the virus.
The interim report deals with 31 of the 34 farms.
The report noted that the DNA sequence of the H5N2-type virus was "almost identical to that confirmed in Guatemala and other countries in Central America."
The ministry said an unauthorized bird-flu vaccine originating in Central America might have been brought into Japan. Instead of immunizing the birds, the vaccine infected them, the sources said.
The report said this theory is backed by the fact that the areas of infection were limited mainly to southern Ibaraki Prefecture.
A U.S. expert's opinion is also included in the report. The expert says the Central America-originated vaccination might have been brought into Japan through a Southeast Asian country hard hit by the highly virulent avian flu.
The ministry will conduct further investigations to pinpoint the source of infection, as well as any use of unauthorized vaccines.
According to ministry officials, the spread of the virus might have been caused when chicken droppings from one infected farm was transported to others in Mitsukaido for compost and other purposes.
The report cited four other possible routes: poultry farm employees who visited shops or other places and left behind the virus; reporters traveling from infected farms; indirect contact between chicken-farm employees and customers at egg-selling stands; and the transport of chickens.
Reporters had visited a poultry farm in Bando in Ibaraki Prefecture just after the Mitsukaido case came to light. Genes of the avian flu virus were also confirmed at the Bando farm, the report said. (IHT/Asahi: November 10,2005)
http://www.cbs13.com/topstories/local_story_075122330.html
CA Company Recalls Measles Vaccines Sold Overseas
(AP) EMERYVILLE, Calif. The Bay Area biotech company linked to recent flu shot shortages said Thursday that it was recalling and withdrawing a measles mumps and rubella vaccine it supplies to developing countries and Italy because of a higher rate of adverse effects than similar vaccines.
Chiron Corp. said about 5 million doses of the Morupar vaccine were distributed to developing countries in 2005, and about 450,000 doses were distributed in Italy. The company said the side-effects included fever, allergic reactions and glandular swelling, which occurred just after inoculation. Chiron said in a statement the reactions do not indicate any long-term risk and that the recall and withdrawal were a precaution. The recall does not affect its other vaccines, Chiron said.
Emeryville-based Chiron set off a public health panic in 2004 when it failed to deliver half the nation's expected 100 million flu shots after British regulators discovered contaminated vaccine at its Liverpool, England, manufacturing plant.
Last fall, Chiron Corp. again said it wouldn't be shipping as many flu shots as it initially had hoped, saying production delays and decreased output occurred after it made repairs at its Liverpool plant. Chiron has said it delivered 13 million flu vaccine shots in 2005, well below the 18 million to 26 million doses it had initially planned to make. Chiron said it will work closely with the World Health Organization to conduct a risk-benefit analysis to see if the measles, mumps and rubella vaccine should be rereleased in limited quantities.
The recall prompted the company to lower its fourth-quarter results by 3 cents per share.
The company revised its earnings to $138 million, or 68 cents per share, from $144 million, or 71 cents a share, for the fourth-quarter, and to $180 million, or 94 cents per share, from $187 million, or 97 cents a share, for the year. Chiron reported results in late January. The Morupar vaccine booked $10 million in sales for 2005. Chiron wrote off about $6 million of Morupar inventory for 2005 and recorded $1.7 million of reserves for anticipated product returns. Shares of Chiron fell 6 cents to $45.64 in midday trading on the Nasdaq Stock Market. The Federal Trade Commission has approved the company's acceptance of a $45-per-share buyout offer from Swiss drug maker Novartis AG.
Novartis already owns 44 percent of Chiron and has offered $5.1 billion in cash for the rest of the stock. The two companies hope to close the deal in the first half of this year, but four shareholders owning a combined 17.5 percent of the company oppose the deal as too cheap. One of those shareholders, ValueAct Capital, said Wednesday it will call for the ouster of Chiron Chief Executive Howard Pien if he backs Novartis' bid and the shareholders turn it down.
"If Howard Pien is going 'on the road' to argue for the appropriateness of the Novartis offer, we can only conclude that our confidence in him has been betrayed, and that he has chosen not to accept responsibility for maximizing shareholder value," ValueAct partner G. Mason Morfit wrote in a letter to Pien and the Chiron board.
(© 2006 The Associated Press. All Rights Reserved. This material may not be published, broadcast, rewritten, or redistributed
REUTERS Health
SOURCE: Morbidity and Mortality Weekly Report, February 23, 2006.
Mumps outbreak at camp among immunized children
NEW YORK (Reuters Health) - In 2005, an outbreak of mumps occurred in a New York summer camp despite the fact that all of the children involved had adequate vaccination coverage, according to the Morbidity and Mortality Weekly Report.
The camp, which had 541 staff members and campers, was in session between June 28 and August 18. Thirty-one cases of mumps were identified, for a 5.7 percent attack rate, Dr. K. Henry, from the Sullivan County Health Department, and associates report.
The first case was in a 20-year-old unvaccinated man who arrived from the UK, where a mumps epidemic was ongoing, to work as a counselor at the camp. His first symptoms, which were seen on June 30, included inflammation of the left salivary gland, sore throat and low-grade fever. However, he continued to work among the camp population.
Between July 15 and July 23, twenty-five more cases of swollen salivary glands were identified, but no diagnoses of mumps were made until July 24. Twelve cases were among campers between 10 and 15 years old, all of whom had been vaccinated with two doses of measles-mumps-rubella (MMR) vaccine after their first birthday. The 19 cases among staff members included nine individuals from the UK, five from the US, three from Australia, and two from Germany. Vaccination was documented for only eight of those affected. Henry's group notes that symptoms, illness duration, and complications were the same for vaccinated and unvaccinated individuals. Although generally a mild and self-limited viral infection, mumps can lead to meningitis, encephalitis, pancreatitis, deafness, inflammation of the testes, and spontaneous abortions among pregnant women.
Therefore, the authors recommend that camp counselors be vaccinated against vaccine-preventable diseases such as mumps. "To prevent large outbreaks of mumps in their communities," the investigators recommend that "US health-care providers should suspect mumps independent of vaccination history, diagnose mumps by using laboratory testing, and report mumps immediately to local health authorities."
Thanh Nien News | Health | Vietnam orders ban after UK-made children vaccine proves deadly
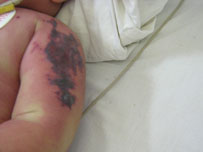
Vietnam orders ban after UK-made children vaccine proves deadly
Serious symptoms in a child as Priorix using complications
The Vietnamese health ministry has ordered a nationwide ban on Priorix, a vaccine for children imported from the UK, after one child died and five others are in critical condition following inoculation.
Assistant Professor Dr Trinh Quan Huan, deputy minister of health, said his ministry had asked the national vaccine analysis institute to analyze the serum which is used against measles, mumps, and rubella.
The six victims are from Ho Chi Minh City’s District 5. The worst affected is Ng.Thi.B, 13 months, who was admitted to the city’s Pediatrics Hospital No 1 Wednesday in medical shock and with breathing difficulty. She had swelling and sores at the injection area and her temperature had shot up to 40oC (104oF). She died the same day. B’s relatives said that half an hour after the vaccination, the injected arm swelled, followed by the chest. Ng.M.Q, also 13 months, was hospitalized with the same symptoms plus diarrhea and convulsions. However, the child is out of danger and doctors are now purifying his blood. The doctors said the two patients had complications caused by Priorix which affected their hearts, brains, and livers. Doctor Le Truong, director of the district health center, said the district had been sent a batch of 109 Priorix doses, 76 of which had been used. “Never before have there been any complications from children’s vaccination like this one,” a veteran doctor said. Priorix is produced by the UK’s GlaxoSmithKline and distributed by Zuellig Pharma.
Reported by T.T., L.C. – Translated by Hoang Bao
Story from Thanh Nien News
Published: 12 May, 2006, 12:56:48 (GMT+7)
Copyright Thanh Nien News
I wonder how long it will take for the vaccine to be cleared from all wrong doing....Deaths Halt Flu Vaccinations in Israel
http://www.washingtonpost.com/wp-dyn/content/article/2006/10/22/AR2006102201220_pf.html
The Associated Press
Sunday, October 22, 2006; 10:55 PM
JERUSALEM -- Israel's Health Ministry halted the administration of flu vaccines on Sunday after at least three people who were inoculated died, a spokeswoman said.
Israeli media reported a fourth person had also died, but officials have not yet confirmed it.
The ministry was investigating whether the deaths are connected to the vaccines, said spokeswoman Inbal Jacobs.
Israel Radio reported the three people who died during the past few days were from the southern Israeli town of Kiryat Gat, and that all had previously suffered from illnesses.
"They were suffering from various illnesses, so that the team that has checked it so far has not found any connection between this tragedy and the vaccinations," said Heath Minister Yaakov Ben-Yizri.
Later Sunday, Israeli media reported a fourth person died after receiving a flu shot _ a man in another part of the country who was suffering from a chronic illness.
The vaccines were manufactured by the French pharmaceutical company Sanofi-Aventis and distributed by the Israeli company Medici. Israel's Army Radio reported that the health ministry had asked the pharmaceutical company to investigate its laboratories.
Israel's Channel 10 TV reported from a health ministry briefing that it was very unlikely that the deaths were related directly to the vaccine, and flu shots might be resumed as early as Monday.
LESS THAN A DAY!!!
Last update - 01:42 23/10/2006
Health Minister: No direct link between flu shots and deaths
By Ron Reznik, Haaretz Correspondent and Haaretz Service
The Health Ministry has stopped the administration of flu vaccines after four people died this week and last week shortly after receiving the inoculations. Three of the four individuals were inoculated at the Leumit Health Maintenance Organization (HMO) in Kiryat Gat. All three suffered from several chronic ailments, including heart disease and diabetes.
The fourth recipient, a 67-year-old man, was insured at the Meuhedet HMO in Petah Tikva and suffered from serious heart disease. He was given the shot on Thursday by his wife, a dentist. A few hours later he was found dead on a city street.
The four persons who died are Shimon Amar, 76, from Kiryat Gat; Yitzhak Azoulay, 68, Kiryat Gat; Nadav Yerushalmi, 53, Moshav Shekef; and Ziggo Kalenstein, 67, of Petah Tikva. The Health Ministry said Sunday that all four were inoculated with vaccine from the same series and purchased from the same manufacturer, the French pharmaceutical firm Sanofi-Aventis, which markets the vaccine all over the world.
Health Minister Yacov Ben-Yizri said Sunday the ministry had asked Sanofi-Aventis whether unexplained fatalities had been reported in any other country. An answer is expected today, and if no other reports had come in, the HMOs would be allowed to continue inoculations, Ben-Yizri said.
The shots were being given last week only to patients at high risk from heart, lung or respiratory disease, high blood pressure, cancer or AIDS.
The decision to call a halt to the inoculations was made by Ben-Yizri and ministry heads Sunday afternoon, after the Kiryat Gat HMO reported the three deaths.
Two of the three were inoculated last Sunday. One died a day later, and the second over the weekend. The third person was inoculated on Monday and died three days later.
The report of the fourth death came while Ben-Yizri was giving a press conference, a few minutes after he told reporters "no connection has been found between the tragedy and the flu inoculations."
The three men from Kiryat Gat who died had all been inoculated in previous years.
The Health Ministry has started an investigation into possible sources of contamination but so far none has been found in the vaccine; some 140,000 people were inoculated over the last week. The Health Ministry also investigated the medical team at the Kiryat Gat clinic. THe shots were administered to all three Kiryat Gat recipients by the same nurse.
The vaccines were supplied to the clinic after the Health Ministry conducted its usual standards and quality tests.
Ben-Yizri noted that the decision to halt the inoculations was taken out of "great sensitivity and concern for the maximum safety of those receiving inoculations."
However, he added, ministry experts had advised him to order the renewal of vaccinations Sunday.
In trying to find any connection between the Kiryat Gat recipients and in response to a question from Haaretz, Dr. Yitzhak Berlovitch, deputy director of the Health Ministry, said "the three were vaccinated by the same nurse, although over a period of days."
Berlovitch, who is also head of the ministry's medical administration, said no cases have ever been documented either in Israel or abroad of people dying after flu inoculations. The deaths could also be explained as being due to the chronic illnesses from which they suffered, he said.
Berlovitch said no autopsies had been carried out because "the Health Ministry has no justification for doing so."
Apparently no additional testing of the vaccine series will be carried out.
The head of the national center for disease monitoring in the Health Ministry, Professor Manfred Green, on Sunday tried to provide a statistical explanation for the four deaths: "About 100 people die every day in Israel from chronic diseases. These four individuals could be part of that group."
The Health Ministry said it would open a hot-line jointly with the HMOs today, from noon until 2 P.M., to calm the public by answering questions about inoculations.
The ministry said anyone who received a flu shot and felt unwell should see his or her family doctor.
A total of 1.2 million vaccine doses were purchased ahead of this year's winter flu season, to combat the five strains of flu against which the World Health Organization and the Health Ministry recommends inoculation: A, B, Caledonian, Wisconsin and Malaysian. Only about a 10th of all inoculations needed by the public are available at present because of delays in growing the virus cultures by the two major vaccine manufacturers in the U.S. and France. The Health Ministry therefore recommends the shots first be given to those whose chronic diseases put them at high risk of complications from the flu, first and foremost the elderly and the chronically ill.
National Jabs Scandal Exposed
Sure shot? ... some children 'got 30 vaccines instead of 13'
By JANE SYMONS, Deputy Health Editor
http://www.thesun.co.uk/article/0,,2-2006510771,00.html
CHILD immunisation records are in such chaos that health chiefs across Britain have no idea what jabs have been given to hundreds of thousands of kiddies. The scandal is exposed in top secret documents leaked to The Sun, which reveal records held by NHS officials are a farce. Last night there were fears that some doctors may even have deliberately fiddled the number of shots given to youngsters - so they can claim huge bonuses. According to the official figures:
Up to 60 per cent MORE shots have been given to children than should have been. Some youngsters appear to have received THIRTY jabs - when they should have had just 13. Amazingly some children are shown as having nine jabs in a single DAY. Doctors are paid whopping bonuses of more than £10,000 if they hit high vaccination targets.
But the shambolic records - dating back an astonishing TWENTY years -are mainly blamed on incompetent paperwork in surgeries. The fiasco was uncovered as part of nationwide moves to transfer medical notes to a central NHS computer. Seventy per cent of batch numbers - the serial numbers used to identify vaccines - were found to be missing. That means it is impossible to trace youngsters who may have received suspect doses.
Six records, taken at random, were ALL found to contain errors. The Sun has been told that Health Minister Caroline Flint was given a top level briefing on the emerging scandal yesterday. It focused on figures taken from Primary Care Trusts in London - but experts believe they are the tip of the iceberg. An insider told The Sun: "It's a complete mess. They don't even know what they don't know. "Records for one borough are in such a poor state they could not be migrated on to the database.
"It looks like records for another 15 Primary Care Trusts are just as bad."
That means records for one million children in London alone are involved.
But the insider said: "There's no reason to think the rest of the country is any different. It's just that London is the first place any of this work has been done." Yesterday afternoon a spokeswoman for Ms Flint initially claimed she had not viewed the document seen by The Sun. The official, who refused to comment on whether Ms Flint had been briefed on its contents, insisted: "We have not had any document." She added: "We would investigate any concerns if they were reported to us." But according to our source the 11am presentation at the Department of Health HQ was made by Richard Granger, the NHS's director general of IT.
Mr Granger is also chief executive of NHS Connecting for Health - which would investigate any fraud. Late yesterday a second spokeswoman confirmed: "Senior officials in the Department of Health, Health Protection Agency and Connecting for Health are working together to sort out this problem." She said Primary Care Trusts had been told to audit records - and ask GPs to check central records against those held at surgeries.
She said of the fear figures had been deliberately fiddled: "That's one conclusion that can be drawn." The innocuously titled document - Summary of Data Quality Issues - states: "There are 50 to 60 per cent more vaccines recorded as being administered as would be expected."
It acknowledges that entries may have been duplicated. But because of the system for paying GPs, doctors have a built-in incentive to give as many jabs as possible. Lawyer Peter Todd, a solicitor who at one time represented 2,000 families who believed their children had been damaged by jabs, said: "If it's wholesale fraud, that should be investigated."
He told how he went through GPs' records as part of his own investigations - and was shocked by some of them. Mr Todd said: "It's fair to say the record keeping is highly variable. Some GP practices are much more organised about it than others. Some are, quite frankly, diabolical." The lawyer added: "The errors relating to batch numbers cause a lot of problems for parents pursuing compensation.
"It's crucial that we know clearly what the child has been given." Under Government guidelines, infants under one should have seven vaccinations against diseases like meningitis, diphtheria and tetanus. But many youngsters are recorded as having vaccines they do not need. By the age of three, children should have had 13 jabs - with no more until they start school. Sun GP Dr Carol Cooper said any child given nine jabs in a day could suffer from fever, convulsions or brain damage.
She said: "It's unlikely to be life-threatening. But if there were an immune system problem - and you don't always know - it's possible that the dose would be too much for them." Dr Cooper said of the dodgy records: "I don't think any parent would allow their child to be vaccinated nine times in a day. "I wouldn't trust the records, it sounds like data has been input more than once. Whatever way you look at it, it's very worrying."
She said she could understand how vital batch numbers could be left off records. Dr Cooper said: "When you give a vaccination you immediately dispose of the needle and vial in a sharps box. It's quite easy to check the serial number and dose, but forget to record this. "Once the sharps are disposed of there is no way of knowing the batch number."
These are the shots children SHOULD have had:
Under 12 months - babies should be immunised against diphtheria, tetanus, meningitis C, polio, whooping cough, pneumococcal infection and Hib - a form of meningitis.
One year old - they get booster jabs for meningitis C and Hib.
Around 13 months - they get their first three-in-one measles, mumps and rubella jab and a final dose of pneumococcal jab.
Ages 3½ to five - booster jabs for diphtheria, tetanus. whooping cough and polio plus a separate MMR booster.
Age 13 - another booster against diphtheria, tetanus and polio. Parents hold their own personal "red book" listing infant vaccinations. Dr Cooper advised any parents worried about the shots their children have had to ask their GP practice to check their records match those in the personal record.
Vaccine death: maker quizzed
Evening Telegraph, UK
10/31/2006
Click here for the URL:
The Procurator Fiscal’s office in Dundee, investigating the death of a woman following her devastating reaction to a flu jab, is seeking answers from the German pharmaceuticals company that made the vaccine (writes Bruce Robbins). Depute fiscal Kirsty McGowan said the firm has been asked if it is aware of any other adverse reactions to the vaccine. Dundee woman Sylvia Thomson suffered a fatal reaction to the injection in November last year and died a few weeks later, on December 2, 2005.
The former PE teacher, who’d been bothered by an intermittent chest infection, asked her GP for the jab as a precaution. A week later, she was taken into hospital suffering from encephalitis — swelling in her brain.
Hospital officials have since admitted that other people may be predisposed to a similar reaction.
Mrs Thomson’s husband, Robert, has been pushing for a fatal inquiry into his 56-year-old wife’s death and is critical of the progress the fiscal’s department has made into the investigation.
However, Ms McGowan told the Evening Telegraph she would be taking the matter further if she didn’t hear back from the company in two to three weeks.
She added, “We have received a fax from one of the vaccine companies saying they have referred our inquiry back to the parent company in Germany. Solvay Pharmaceuticals is German-owned and has a base in Holland. They have to provide us with a full answer and that couldn’t be done by the distributors in Britain.
“We’ve identified the batch numbers of the vaccine concerned and are waiting for a report from the company. We are looking for other adverse reactions to the vaccine and the company will have all those details.”
Ms McGowan rejected Mr Thomson’s claims the matter hadn’t been given a high enough priority and said she had updated him at the beginning of October.”
Mr Thomson said he believed the fiscal’s department had spent a lot of time speaking to Mrs Thomson’s GP and a doctor at Ninewells Hospital when they should have been on to the drugs company straight away.
“There must be a Government body that approved the vaccine for use in the UK and the fiscal could have contacted them straight away to find out what tests had been carried out on it before it was made available.
“There was a report out recently saying that it couldn’t be proved that ‘flu vaccines do any good. Here we are heading into the vaccination season and we still don’t know if this particular vaccine is going to stop people getting the ‘flu or end up killing others.”
Vaccine leaves 700 kids at risk
By Ann McGlynn | Thursday, November 23, 2006
(1) Comments | Rate this article | Default | Large
About 700 children who received inoculations at the Community Health Care clinic in downtown Davenport will need to be revaccinated after state inspectors found problems with a refrigerator used to store the doses.
The children and their parents or guardians have been contacted by letter, said George Barton, CEO of the clinic. About one-third of those impacted already have come in for the replacement shots. There is no cost for the families to receive the revaccinations.
Barton called the move “precautionary.” The children, he said, “won’t get sick. They just may not be fully immunized” because improperly stored vaccine can be less effective.
Repeating a vaccination is not harmful, Barton said in a letter sent to parents from himself and Syed Haque, medical director at Community Health Care, or CHC.
The children impacted are those who received their shots at the clinic on River Drive in Davenport, not any of the other CHC clinics, over the past several months, Barton said.
“Our goal is to ensure that our patients are protected from disease,” he added. “We are taking every step necessary to ensure that families are contacted and come back to get the needed shots. We have also set up safeguards to prevent this type of situation from occurring again.”
Specifically, CHC has purchased new refrigerators and freezers for all of its clinics that provide vaccine, he said. The appliances have internal thermostats that measure temperature four times a day.
Vaccines must either be kept in a refrigerator with the temperature set between 36 and 46 degrees or a freezer with a temperature of at least 15 degrees below zero, said Roma Taylor, clinical services director for the Scott County Health Department. The department is working with the clinic to resolve the vaccination issue.
The work included reviewing the children’s medical charts to determine who needs to return to the clinic for additional vaccine and who does not, she said.
State inspectors discovered the temperature problem during a routine inspection, Barton said.
The state does the inspections to ensure the vaccine remains effective, said Kevin Teale, a spokesman for the Iowa Department of Public Health. The state has handled a couple of similar cases, including an instance at a Cedar Rapids clinic three years ago and another now under investigation in western Iowa.
“This doesn’t mean the vaccine is totally ineffective. It just may not be as effective as it should be,” Teale said, adding that the state encourages clinics and doctor’s offices to take the required logging of temperatures seriously.
Ann McGlynn can be contacted at (563) 383-2336 or amcglynn@qctimes.com.
High school students may have received ineffective vaccine
By Amanda Karr
The Daily Reflector
Friday, March 02, 2007
More than a hundred students at Ayden-Grifton High School will need an extra stick of the needle to ensure they're vaccinated against meningitis.
On Feb. 19, 134 students at the school participated in the Pitt County Health Department's Menactra Vaccination Campaign. Health department officials have since discovered the vaccine given to students that day may not be effective.
Turns out, the vaccine was being stored in a refrigerator at an incorrect temperature.
The local health department consulted officials with the North Carolina Immunization Program in Raleigh and the Centers for Disease Control and Prevention decided to recommend students repeat the vaccine to ensure they are adequately protected.
"The vaccine that these students received poses no threat to the student's health," said Dr. John Morrow, Pitt County health director. "The concern is that the students may not be adequately protected against meningococcal meningitis."
Meningitis is an infection of the fluid surrounding the spinal cord and brain. It can be fatal. Some forms of bacterial meningitis are contagious.
The vaccine being administered by the health department protects against four types of meningoccal disease, including two of the three most common in the United States, according to the Centers for Disease Control. Many colleges recommend the vaccine for incoming students who plan to live in the close quarters of a dorm setting.
Reaching those students is the goal of the health department's vaccination campaign, said Jo Morgan, the department's health educator. The shots are free as part of the campaign.
Tuesday, health department officials began calling the parents of the Ayden-Grifton students who received the vaccine earlier this month and Wednesday mailed letters to explain the situation, Morgan said.
Another date is being scheduled to re-administer the vaccine.
The vaccines administered at clinics held at North Pitt, J.H. Rose and D.H. Conley high schools are not believed to have been compromised, Morgan said.
The health department is establishing additional safeguards to prevent similar future problems, including setting up a system that will check the refrigerator temperature daily, Morgan said. There already is an alarm system on the refrigerator that is supposed to alert workers if something is wrong.
"We have a good system in place. We just fine-tuned it some more," Morgan said.
She said the health department has three refrigerators, and the problem caused no major losses of health department supplies.
Amanda Karr can be contacted at akarr@coxnc.com and 329-9574.
Find this article at:
http://www.reflector.com/local/content/news/stories/2007/03/2/vaccine.html
Russian Prosecutors Launch Probe Into Vaccine Tests on Children
Created: 04.03.2007 12:48 MSK (GMT +3), Updated: 13:21 MSK, 16 hours 16 minutes ago
http://www.mosnews.com/news/2007/03/04/toddlers.shtml
MosNews
Russian prosecutors are investigating a local hospital on suspicions it illegally tested vaccines made by GlaxoSmithKline Plc on toddlers, the Reuters news agency reports.
Europe’s biggest drugmaker denied the claims and said there was no evidence of adverse events or misconduct in the way the study had been carried out.
Glaxo vaccines were tested on more than 100 children between one and two years of age at the hospital in Volgograd after Russian health authorities approved the trials in 2005.
But prosecutors claim parents were not properly informed and they thought these were routine vaccinations.
According to the prosecutors, Glaxo paid the clinic in southwestern Russia $50,000 to conduct the trials, which made some children ill.
“According to the contract, only healthy children can take part in this experiment,” said a spokeswoman for the Volgograd region prosecutors, Lydia Sergeyeva.
“In this case all children were sent for trials, healthy or unhealthy, and many of them had been diagnosed with diseases.
”They had no right to put children with health problems through these clinical tests because ... it can lead to a deterioration in the child’s condition, as happened with one girl for instance.“
Sergeyeva told Reuters of a 2-1/2-year-old girl whose neurological illness progressed sharply after she was vaccinated. The girl can hardly speak and shows other signs of arrested development, she said.
Glaxo said the tests were part of a wider clinical trial programme involving 5,700 children across Europe, including around 1,000 in Russia.
The project is designed to evaluate the effectiveness of different vaccines against varicella, or chickenpox, and involves Glaxo vaccines that are already approved for use.
A company spokeswoman said its own internal audit showed informed consent had been given by all parents and doctors involved in the trial had reported no signs of adverse effects.
”GlaxoSmithKline is extremely concerned about unsubstantiated and untrue allegations circulating related to the clinical trial in Russia,“ she said.
The prosecutors allege the clinic had no right to conduct the tests as it was not a state clinic.
When asked why the tests were nonetheless allowed in the Volgograd hospital, Sergeyeva said it was a question for the authorities who granted the permission.
A regional court ruled last month that the vaccinations should stop but Sergeyeva said the hospital promised to appeal. If no appeal is made, all future tests of the vaccines in the clinic will be banned as of next week.
http://www.bradenton.com/mld/bradenton/news/local/16984402.htm
Last-minute effort gets vet closer to new heart
By DONNA WRIGHT
dwright@bradenton.com
AUDIO | Hear Sally Spehr and her son, Navy veteran Andrew Spehr
BRADENTON
- After a rollercoaster week of insurance complications and bureaucratic red tape, Navy veteran Andrew Sperh might get a new heart after all.
An 11th-hour effort by the local Social Security office and the Florida Department of Children and Families helped the Bradenton resident get emergency Medicaid coverage Tuesday to cover the transplant procedure at Tampa General Hospital - if a replacement heart becomes available.
"I am ready," Sperh said in a low voice, reclining in his hospital bed at Blake Medical Center, his sunken cheekbones revealing the toll congestive heart failure has taken on his 26-year-old body. The 1999 Bayshore High School graduate is a shadow of his former self. When discharged from the Navy two years ago, Sperh weighed in at more than 200 pounds. Today, he's closer to 140, according to his doctors.
Although the Department of Veterans Affairs would cover the transplant, Andrew and his parents, Sally and Kurt, didn't want to go through Bay Pines Veterans Hospital in St. Petersburg because they feared Andrew would meet too many delays caused by the sheer size of the federal health care system. And Dr. John K. Lourie, the Bradenton cardiologist who diagnosed Sperh's congestive heart failure last February, said the former sailor has no time to spare.
Without a new heart, Sperh could die, said Lourie who described the difference between care in veterans hospitals and private hospitals as "night and day."
"It's not a questions of quality but of timing," he said. "You have much longer waits through the VA."
And time is running out, said Bradenton attorney Eddie Mulock, who has had four transplants, including a new heart.
"People don't understand the significance of delays," said Mulock, who now advises the Sperhs. "Any delay can cause liver, lung and heart functions to decrease. Before Andrew can get a new heart, they will test him, and if his organs don't meet the function standards for transplant, they may disqualify him, even if a heart is available." As time passes, the odds of organ failure increase because Sperh's enlarged and damaged heart cannot keep life-giving blood coursing through his veins, Lourie said.
Adverse reaction?
One thing is certain: Andrew Sperh himself has been the victim of unfortunate timing through this whole ordeal, dating back to his discharge physical in the spring of 2004. Just as he was about to leave the Navy, orders came for the USS Spruance, the destroyer that had been Sperh's home for four years, to sail to the Middle East. Sperh's talents as a navigator were needed for the voyage. The Quartermaster 3rd Class voluntarily extended his service.
On July 1, while his destroyer was plying the Arabian Sea, the crew was ordered to have smallpox vaccines.
Sperh's medical record clearly states that he had an exemption from getting the vaccine because he was due to be discharged.
He had no choice.
"They told me if I refused, I would be court-martialed," Andrew said.
Andrew is convinced an adverse reaction to the vaccine led to his heart disease. Lourie said it's possible.
A study done the the Centers for Disease Control and Prevention revealed 14 cases of myocarditis -Sperh's diagnosis - and one fatal myocardial infarction, or heart attack, were reported among military personnel who received the vaccine between January and March of 2003. Andrew began to experience chest congestion around the time he was discharged, on Sept. 5, 2004. When Lourie looked at Sperh's past X-rays, he found a nine-month progression of congestive heart disease. In the first, taken at Bay Pines Hospital in May 2005, the disease is visible, he said.
The second, taken at Manatee Memorial Hospital three months later, shows the condition worsening.
By the time Sperh was rushed to Blake on Super Bowl Sunday, 2006, he was in serious trouble, throwing up clots of blood and exhibiting signs of severe congestive heart failure.
He's been biding time ever since.
'We are ready'
A week ago, the Sperhs and Lourie thought everything was set.
On Thursday, Sperh went to Tampa General for his first consultation with the cardiac transplant team. His insurance papers were in order, the hospital said. He was accepted for preliminary testing. But that night, after returning to Bradenton, Sperh had another serious attack of congestive heart failure and was rushed to Blake Medical Center.
The next morning, his case manager told the stunned veteran that his United Healthcare coverage through his former employer was terminated effective March 9, the day after his three-month medical leave from his job came to an end. But now, thanks to the work of Kelly Guest at the local Social Security office and Johnyta Kelly at DCF, Sperh is back on track for the transplant procedure.
Once he passes the preliminary tests, he will be put on the list for a new heart, Lourie said.
The timing is auspicious, said Sally Sperh.
"I hate to say it, but this is prom and graduation season when a lot of accidents happen," she said. "It could be that one family's tragedy will turn out to be our miracle. I don't know if I am ready to deal with that." And Sperh's only hope is that a new heart becomes available before his time runs out. "Thank God, we now have coverage," his mother said. "We are ready. As soon as we get the word, we're on our way."
Thanh Nien News, Vietnam
June 16, 2007
Rabies vaccine used in Vietnam unsafe, says local expert - Prof Dinh Kim Xuyen, deputy director of the ministry’s rabies prevention project
A Vietnamese rabies expert urged the Ministry of Health Friday to stop using a rabies vaccine suspected to have caused paralysis in a man who got a shot recently, calling it unsafe. The affected man was diagnosed with acute flaccid paralysis three weeks ago after receiving the sixth of a series of eight shots of Fuenzalida vaccine in a Ho Chi Minh City clinic. Prof Dinh Kim Xuyen, deputy director of the ministry’s rabies prevention project, said the World Health Organization had warned against the use of mouse brain rabies vaccines like Fuenzalida because they were unsafe and prone to side effects.
But they were still being used in Vietnam due to their low cost, she said, adding that 400,000-500,000 people continued to get mouse brain vaccines every year.
In 2002 her agency had recommended that the ministry replaced mouse brain vaccines with the safer cell vaccines. But the ministry had been unable to do so because of finding supplies and the high cost. Instead, it had begun to use both vaccines, the mouse brain Fuenzalida vaccine and the cell Verorab vaccine.
It had also reduced the number of facilities producing the former from three to one, and advised staff at vaccination centers to closely monitor people getting mouse brain vaccines for excessive reaction. Mouse brain vaccines normally caused minor reactions which did not need treatment. But out of 10,000 recipients, they caused paralysis in one to three cases. If doctors had discovered the side effects in the Ho Chi Minh City victim earlier, his paralysis could have been prevented.
Reported by Lien Chau – Translated by The Vinh
Story from Thanh Nien News
- ends -
Nation to distribute safer rabies vaccine, discontinue old version
http://vietnamnews.vnagency.com.vn/showarticle.php?num=01HEA140707
(14-07-2007)
Ha Noi — There is sufficient rabies vaccine in Viet Nam to last till the end of the year with nearly 700,000 doses in stock, according to the Ministry of Health’s Drug Administration.
Deputy Health Minister Cao Minh Quang said that six vaccine providers have sufficient doses in store for this year and that they will import alternative rabies vaccines in 2008.
Verorab, now considered the safest rabies vaccine by the World Health Organisation, is sold at between VND120,000 and VND304,000 per dose. Quang said the ministry has authorised Vaccine and Medical Product Company No. 2 and HCM City Pasteur Institute to import Verorab rabies vaccine in bulk for packaging in Viet Nam. The vaccine price should fall and that the ministry would publicise vaccine prices so that local authorities could select best suppliers, he added.
This means that Viet Nam will be able to discontinue using the potentially dangerous Fuenzalida rabies vaccines.
The Health Ministry had earlier permitted the storage of 300,000 doses of Fuenzalida in case of emergencies or shortages.
Fuenzalida was put under spotlight after a number of people in HCM City were paralysed after being given a dose of the rabies vaccine made using material extracted from the brains of suckling mice.
Health ministry statistics reveal that eight out of 400 people vaccinated suffered severe side effects of paralysis or the sudden onset of encephalitis in 2006. — VNS
Fears were yesterday raised that there is a possibility the disease may somehow have been transferred from vaccines at the Institute for Animal Health in Surrey.
http://scotlandonsunday.scotsman.com/scitech.cfm?id=1224802007&format=print
Vaccine lab link to foot and mouth
EDDIE BARNES AND MURDO MACLEOD
A GOVERNMENT laboratory based two miles from where foot and mouth disease was discovered in a herd of cows is being investigated as the possible source of the outbreak.
Fears were yesterday raised that there is a possibility the disease may somehow have been transferred from vaccines at the Institute for Animal Health in Surrey.
The revelation emerged 24 hours after foot and mouth was discovered in a herd of cows near the village of Normandy, Surrey.
It came as:
• Scottish ministers said they may consider a plan to break away from the UK ban on meat exports imposed by the UK government;
• Vets in London revealed that other farms were being checked for possible infection;
• Tourist chiefs and officials said that Scotland remained "open for business".
The institute in Surrey houses material used to make vaccines for outbreaks of foot and mouth. To do so, strict regulations are laid out to keep the disease secure.
Chief veterinary officer for England and Wales, Dr Debby Reynolds, announced that it was coming under scrutiny.
The country's leading bacteriologist, Professor Hugh Pennington, said last night that the institute must now be seen as a possible cause of the source of the outbreak. Pennington said: "There are lots of other possible explanations, but this is the one which will be looked at. These escapes do happen from time to time.
"That lab has a very, very good record of not having any escapes. It's a first-class lab. When I was rung up to be told there was an outbreak in Surrey, the first thing I did was look up the map and it turned out it was closer that I thought."
A spokesman for Defra, the Department for Rural Affairs, confirmed that the Institute for Animal Health in the village of Pirbright was among the places being examined as a source.
"One of the first actions we took was to ask Pirbright to review and assess their biosecurity arrangements. We are seeking to involve independent experts in this work," he said.
Farmers and rural businesses right across Britain were on tenterhooks last night, fearing that further outbreaks of the disease would be announced.
In 2001, a nationwide outbreak led to the slaughter of between 6.5 million and 10 million animals, ruined many farmers and rural businesses and is estimated to have cost the country up to £8.5bn.
Britain's voluntary ban on all meat exports will now last for a minimum of three months, cutting off the £1m-a-month Scotch beef export market.
But ministers in Edinburgh say they are looking at whether Scotland can be withdrawn from the ban, if the outbreak remains in the south. Cabinet Secretary for Rural Affairs Richard Lochhead said: "There is the ability for regionalisation".
Meanwhile, Britain's chief vets said they were considering whether mass vaccination may be required some time this week.
In 2001, officials decided against vaccinating animals, a move which led to the mass cullings. However, with new laws in place, they are now legally obliged to consider vaccinations.Charles Milne, Scotland's chief veterinary officer, said: "We have already activated vaccine teams, so they will be in place within five days, should vaccination be the central policy."
Vaccinations would be controversial as they would extend the EU ban on exports to six months.
But farming leaders last night said they would be content to work with whatever solution the vets thought best.
James Withers, deputy chief executive of the National Farmers Union in Scotland, said:
"Our message is crystal clear - vigilance is key. Farmers are in and around their stock and that is enough to check on the symptoms of the disease. Farmers have it in their power to restrict the opportunities for disease."
Prime Minister Gordon Brown said authorities were doing "everything in our power" to avoid a repeat of the catastrophe six years ago. He returned from holiday yesterday to chair a meeting of Cobra, the government's emergency committee. Afterwards, he held discussions with First Minister Alex Salmond about the implications for Scotland. Ministers stressed once again that the disease had no implications for human health.
After a 3km protection zone was thrown around the affected farm in Surrey, authorities turned their focus on a desperate hunt to quickly find the source of the disease.
The spread of the disease in 2001 was largely blamed on the slow response to first reports which allowed infected animals to come into contact with other animals from all parts of the country.
The hope last night was that the outbreak would be contained to the one farm. But Reynolds said officials were investigating other possible outbreaks of the disease.
She said a small number of potential cases had been reported in the wake of the discovery on the farm near Guildford, Surrey. A spokesman for the Scottish Executive said it was not aware that any cases had yet been reported north of the Border.
Conservative leader David Cameron, who postponed a planned holiday in Brittany in order to keep abreast of the developing situation, said: "We must do everything possible to make sure this is not a further blow to farming." He added: "The other great lesson from 2001 is that it was not just farmers that suffered but also shops, holiday cottages, pubs and hotels.
"Farmers and rural communities should know that the whole country is on their side."
Donald Biggar, chairman of Quality Meat Scotland, said: "We are holding our breath to see what emerges in the next 48 hours. Everyone is just hoping and wishing that it won't be a repeat of 2001, which was disastrous. Whatever happens this will have a major impact and be a disruption to trade. We are going to lose our exports instantly and it will take a while to recover them.
"It's come at a time when our beef exports were building up nicely. We've only been back in the European market for 15 months having got the BSE business out of the way."
Executive minister Lochhead added: "Foot and mouth disease is clearly a serious disease of livestock and sadly its shadow is now looming over Scotland and our rural life once again. The key objective of the Scottish government is to ensure that any such disruption is kept to an absolute minimum."
He added: "Let me be clear - Scotland's countryside remains open. That's an important message here at the heart of our summer season here in Scotland."
Lochhead and Salmond will today attend the Turriff agricultural show, in a bid to show their backing for the rural economy.
Thanh Nien News | Society | Man sues after vaccine paralyzes him
Man sues after vaccine paralyzes him
A man in the southern province of Hau Giang has sued a local health agency and vaccine maker, claiming a vaccine paralyzed him last year. Tran Minh Chi, 37, is asking for VND85 million (over US$5,300) from the Phu Huu Commune Health Center and the Vaccine and Bio-Medical Product Company No. 2, saying a rabies vaccine manufactured by the firm paralyzed him. On August 2 last year, Chi received his first Rabivax II shot at the center after being bitten by a stray dog. On August 12, he received the sixth in a course of eight shots of the vaccine. The next day, he became paralyzed and was rushed to the Can Tho General Hospital and later to the Cho Ray Hospital in Ho Chi Minh City. He has endured quadriplegia since then. In his lawsuit, Chi said the center failed to inform him of the disadvantages of the vaccine so that he could have chosen a better one. Meanwhile, in documents sent to the court, the health center denied responsibility concerning the man’s paralysis and attributed the mishap to the producer, saying that its vaccine failed to meet quality standards.
The company also denied its involvement in the case.
The firm said all the information related to the vaccine was included with the medicine.
The vaccine is known to cause marrow paralysis to one out of every 10,000 people inoculated by the shots.
The Ministry of Health halted the production of Rabivax II in September last year following a similar case in May in which a HCMC man had suffered acute flaccid paralysis due to the same vaccine. The company said the shots given Chi had been produced before the deadline, which meant it had no responsibility in his mishap. A hearing will take place next Wednesday, according to judge Ngo Thi Hien. Chi filed his suit at the Chau Thanh District People’s Court.
Reported by Mai Tram
http://www.thanhniennews.com/print.php?catid=3&newsid=35905
Story from Thanh Nien News
Published: 20 February, 2008, 14:07:06 (GMT+7)
Copyright Thanh Nien News
Children got wrong immunizations, including cervical cancer vaccine, at Salem County clinic, officials say Print Email By Bill Gallo Jr. | For NJ.com
Email the author | Follow on Twitter
on July 04, 2015 at 9:52 AM, updated July 04, 2015 at 2:06 PM Stay connected to nj.com ×
Reddit
SALEM — At least five children at a Salem County-run clinic were given improper immunizations, including one who received a cervical cancer prevention vaccine, officials say.
An annual state compliance audit of the Shots For Tots program "identified irregularities respecting its appraisal of standards, policies and competencies regarding the clinic operation, patient records, vaccine storage and medication dispensing," according to a statement released by the county this weekend.
"As soon as it was brought to our attention we reacted to this finding," said Salem County Freeholder Director Julie Acton on Saturday. "Our main concern is the safety of our residents. It's sad and disturbing when something like this takes place."
In addition to the children receiving the wrong medicine, vaccines with an estimated value of $20,000 used for the Shots for Tots program were deemed unsafe after a refrigeration unit failed and they had to be destroyed.
In the wake of the revelations, Salem County agreed to pay for medical monitoring costs for the five children receiving improper immunizations "for the foreseeable future until their medical status may be determined to a reasonable degree of medical certainty."
The parents will take the children to a physician of their choice for the monitoring officials say.
Meanwhile, the clinic has been shut down. The nursing supervisor and nurse in charge of the clinic have both resigned as employees of the Salem County Department of Health, county solicitor Michael Mulligan said Saturday.
The Shots for Tots clinic had been held once a month at The Memorial Hospital in Mannington Township.
Once the audit revealed problems with the, Salem County Health Department Director Rita Shade ordered a subsequent investigation that included a complete review of the charts of the 22 patients who had received immunizations and an inventory of all vaccines.
Those patients include 20 children and two adults who visited the clinic in the past year. The clinic serves the uninsured.
In what county officials are calling "the most egregious medication dispensing case," one 2-year-old boy received an "excessive dose" of the cervical cancer prevention vaccine Gardasil.
That unidentified child's mother and pediatrician were notified on June 30 about the mistake, officials said.
County officials said "The nature, likelihood and extent of any negative health effects, if any, are not presently known other than that the child may be at risk of neurological damage."
Shade on Saturday called the incident "extremely unfortunate."
She said the five children who received wrong medications included: Two children who were injected with vaccines that had expired; one child received a flu mist dose, but was under the age recommended to receive the mist (used in place of a flu shot); one child who officials are still trying to determine what type of vaccine they received and the one boy who received the Gardasil injection.
As the for vaccines that needed to be discarded, Shade said a compressor on the refrigeration unit where the medicine was stored at the Salem County Office of Emergency Management headquarters failed on June 20.
The vaccines, when needed for the clinic, were transported from the OEM site in Mannington to Memorial Hospital.
It was decided, after consulting with the vaccine manufacturers, that the failure of the refrigeration unit had made the medicine unstable and it should be discarded, Shade said.
After learning of Shade's findings, Acton and Freeholders Bob Vanderslice and Dale Cross, members of the Administration Committee, held an emergency meeting with county Chief Financial Officer Kate Coleman and Mulligan. The freeholders, at the recommendation of Coleman and Mulligan, authorized the payment of the medical monitoring costs.
The audit which originally brought the improper dispensing of medicine to light covered the period from July 1, 2014 to June 30, 2015.
The clinic provided vaccinations of the type that are typically required to attend school.
According to the county website, the Shots for Tots program offered free immunizations for children the second Thursday of every month at Memorial Hospital.
Immunizations were offered for children 2 months to 18 years of age. Parents were required to bring an up-do-date shot record.
Shade said since another provider in the county offers the same services the Shots for Tots clinic did, the county-run clinic has been shut down for now. It will be up to the freeholders to decide whether or not to reopen the clinic.
Bill Gallo Jr. may be reached at bgallo@njadvancemedia.com. Follow South Jersey Times on Twitter @TheSJTimes. Find the South Jersey Times on Facebook.
Email the author | Follow on Twitter
on July 04, 2015 at 9:52 AM, updated July 04, 2015 at 2:06 PM Stay connected to nj.com ×
SALEM — At least five children at a Salem County-run clinic were given improper immunizations, including one who received a cervical cancer prevention vaccine, officials say.
An annual state compliance audit of the Shots For Tots program "identified irregularities respecting its appraisal of standards, policies and competencies regarding the clinic operation, patient records, vaccine storage and medication dispensing," according to a statement released by the county this weekend.
"As soon as it was brought to our attention we reacted to this finding," said Salem County Freeholder Director Julie Acton on Saturday. "Our main concern is the safety of our residents. It's sad and disturbing when something like this takes place."
In addition to the children receiving the wrong medicine, vaccines with an estimated value of $20,000 used for the Shots for Tots program were deemed unsafe after a refrigeration unit failed and they had to be destroyed.
In the wake of the revelations, Salem County agreed to pay for medical monitoring costs for the five children receiving improper immunizations "for the foreseeable future until their medical status may be determined to a reasonable degree of medical certainty."
The parents will take the children to a physician of their choice for the monitoring officials say.
Meanwhile, the clinic has been shut down. The nursing supervisor and nurse in charge of the clinic have both resigned as employees of the Salem County Department of Health, county solicitor Michael Mulligan said Saturday.
The Shots for Tots clinic had been held once a month at The Memorial Hospital in Mannington Township.
Once the audit revealed problems with the, Salem County Health Department Director Rita Shade ordered a subsequent investigation that included a complete review of the charts of the 22 patients who had received immunizations and an inventory of all vaccines.
Those patients include 20 children and two adults who visited the clinic in the past year. The clinic serves the uninsured.
In what county officials are calling "the most egregious medication dispensing case," one 2-year-old boy received an "excessive dose" of the cervical cancer prevention vaccine Gardasil.
That unidentified child's mother and pediatrician were notified on June 30 about the mistake, officials said.
County officials said "The nature, likelihood and extent of any negative health effects, if any, are not presently known other than that the child may be at risk of neurological damage."
Shade on Saturday called the incident "extremely unfortunate."
She said the five children who received wrong medications included: Two children who were injected with vaccines that had expired; one child received a flu mist dose, but was under the age recommended to receive the mist (used in place of a flu shot); one child who officials are still trying to determine what type of vaccine they received and the one boy who received the Gardasil injection.
As the for vaccines that needed to be discarded, Shade said a compressor on the refrigeration unit where the medicine was stored at the Salem County Office of Emergency Management headquarters failed on June 20.
The vaccines, when needed for the clinic, were transported from the OEM site in Mannington to Memorial Hospital.
It was decided, after consulting with the vaccine manufacturers, that the failure of the refrigeration unit had made the medicine unstable and it should be discarded, Shade said.
After learning of Shade's findings, Acton and Freeholders Bob Vanderslice and Dale Cross, members of the Administration Committee, held an emergency meeting with county Chief Financial Officer Kate Coleman and Mulligan. The freeholders, at the recommendation of Coleman and Mulligan, authorized the payment of the medical monitoring costs.
The audit which originally brought the improper dispensing of medicine to light covered the period from July 1, 2014 to June 30, 2015.
The clinic provided vaccinations of the type that are typically required to attend school.
According to the county website, the Shots for Tots program offered free immunizations for children the second Thursday of every month at Memorial Hospital.
Immunizations were offered for children 2 months to 18 years of age. Parents were required to bring an up-do-date shot record.
Shade said since another provider in the county offers the same services the Shots for Tots clinic did, the county-run clinic has been shut down for now. It will be up to the freeholders to decide whether or not to reopen the clinic.
Bill Gallo Jr. may be reached at bgallo@njadvancemedia.com. Follow South Jersey Times on Twitter @TheSJTimes. Find the South Jersey Times on Facebook.
