http://jama.ama-assn.org/cgi/content/abstract/292/20/2478
Guillain-Barré Syndrome Following Influenza Vaccination
Penina Haber, MPH; Frank DeStefano, MD, MPH; Fredrick J. Angulo, DVM, PhD; John Iskander, MD, MPH; Sean V. Shadomy, DVM, MPH; Eric Weintraub, MPH; Robert T. Chen, MD, MA
JAMA. 2004;292:2478-2481.
Context An unexplained increase in the risk of Guillain-Barré syndrome (GBS) occurred among recipients of the swine influenza vaccine in 1976-1977. Guillain-Barré syndrome remains the most frequent neurological condition reported after influenza vaccination to the Vaccine Adverse Events Reporting System (VAERS) since its inception in 1990.
Objective To evaluate trends of reports to VAERS of GBS following influenza vaccination in adults.
Design, Setting, and Participants VAERS is the US national spontaneous reporting system for adverse events following vaccination. Reports of GBS in persons 18 years or older following influenza vaccination were evaluated for each influenza season from July 1, 1990, through June 30, 2003. The number of people vaccinated was estimated from the National Health Interview Survey and US census data. Beginning in 1994, active follow-up was conducted to verify GBS diagnosis and obtain other clinical details.
Main Outcome Measure Reporting rates of GBS following influenza vaccination over time.
Results From July 1990 through June 2003, VAERS received 501 reports of GBS following influenza vaccination in adults. The median onset interval (13 days) was longer than that of non-GBS reports of adverse events after influenza vaccine (1 day) (P<.001). The annual reporting rate decreased 4-fold from a high of 0.17 per 100 000 vaccinees in 1993-1994 to 0.04 in 2002-2003 (P<.001). A GBS diagnosis was confirmed in 82% of reports. Preceding illness within 4 weeks of vaccination was identified in 24% of reported cases.
Conclusions From 1990 to 2003, VAERS reporting rates of GBS after influenza vaccination decreased. The long onset interval and low prevalence of other preexisting illnesses are consistent with a possible causal association between GBS and influenza vaccine. These findings require additional research, which can lead to a fuller understanding of the causes of GBS and its possible relationship with influenza vaccine.
Author Affiliations: National Immunization Program, Centers for Disease Control and Prevention (Ms Haber, Drs DeStefano, Iskander, Shadomy, and Chen, and Mr Weintraub); and National Center for Infectious Diseases, Division of Bacterial and Mycotic Diseases (Dr Angulo), Atlanta, Ga.
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Legal Concerns and the Influenza Vaccine Shortage Mello and Brennan JAMA 2005;294:1817-1820.
Trends in Influenza Vaccination-Associated Guillain-Barre Syndrome Journal Watch Pediatrics and Adolescent Medicine 2005;2005:14-14.
Trends in Influenza Vaccination-Associated Guillain-Barre Syndrome Journal Watch Infectious Diseases 2004;2004:5-5.
Surround H1N1 Vaccine Which May Contain Thimerosal Concerns Surround H1N1 Vaccine Which May Contain Thimerosal July 30th, 2009 Concerns Surround H1N1 Vaccine Which May Contain Thimerosal
By Shaun Heasley
July 30, 2009A swine flu vaccine expected this fall is raising red flags because it will likely contain a preservative some link to autism despite scientific research repeatedly proving otherwise.
Government officials say they hope to have 160 million doses of a vaccine for swine flu, or H1N1, available by the fall when flu season is expected to kick in. A vaccine is currently going through clinical trials. Pregnant women, children and health care workers will be the first to be vaccinated.
Scientists say the swine flu vaccine will be similar to the flu vaccines provided annually with little fanfare. However, some consumer groups are reserved about the new vaccine because of the possibility that it could contain thimerosal in combination with a second additive in an effort to produce large quantities more quickly.
Furthermore, a vaccine offered to combat a different strain of the swine flu in the 1970s is linked to increased incidents of a neurological disorder.
But makers of the vaccine say they anticipate manufacturing two versions, one with and one without thimerosal, which ought to ease some fears, reports ABC News.
Read the Entire Story Here.
Copyright © 2009 Disability Scoop, LLC. All Rights Reserved.
HHS 2009 H1N1 Vaccine Development Activities
Fact Sheet
Overview The newly emergent 2009 H1N1 influenza virus is a novel virus with pandemic potential. Consistent with the National Strategy for Pandemic Influenza, HHS is committing funds for the production of pilot lots for clinical studies, as well as a bulk supply of antigen and adjuvant for use in a potential vaccine for the 2009 H1N1 which will become a part of the national stockpile of pre-pandemic influenza vaccines.
A vaccine is made from a virus or bacteria (referred to as an antigen), which causes the human body’s immune system to develop antibodies against a specific virus or bacteria so the body can recognize and fight the virus or bacteria. Adjuvants may be added to a vaccine to help generate a stronger immune response so less vaccine is needed for the body to recognize and fight a virus or bacteria.
Vaccine Development When the 2009 H1N1 strain was isolated and identified as a novel influenza virus, work began to prepare a virus reference strain. This is a standard practice when new influenza strains are discovered, where a clinical sample of the virus is mixed with another influenza virus that grows in eggs to develop a new virus that has some of the properties of the novel virus and the ability to grow in eggs. This work is necessary in order to create an influenza vaccine using conventional methods.
Once a virus reference strain is ready, it will be made available to influenza vaccine manufacturers in order to create a master virus seed, which prepares a virus to be used in making the vaccine.
HHS Contracting Activities Since 2004, HHS has contracted with manufacturers that currently hold U.S. licenses for flu vaccine as part of the National Strategy for Pandemic Influenza. In May 2009, HHS issued new orders on these contracts to produce a bulk supply of vaccine antigen and adjuvant and to produce pilot (also called investigational) lots of a 2009 H1N1 vaccine. Most will be stored in bulk, and a small amount will be prepared as vaccine for use in clinical studies to evaluate vaccine safety and the dosage required for a protective effect. This research will include studies with adjuvant to determine its safety and the effect it would have on the immune system’s response.
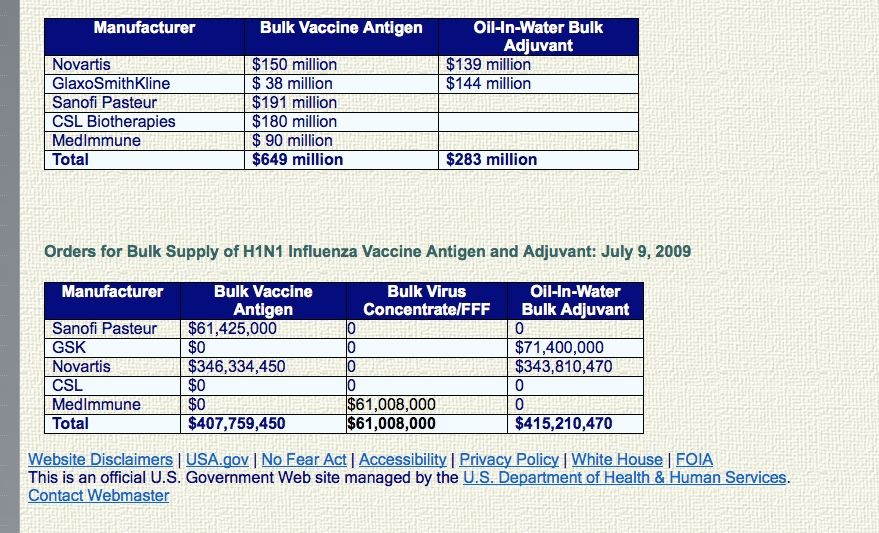
Orders for Bulk Supply of H1N1 Influenza Vaccine Antigen and Adjuvant: May 22, 2009
Guillain-Barré Syndrome Following Influenza Vaccination
Penina Haber, MPH; Frank DeStefano, MD, MPH; Fredrick J. Angulo, DVM, PhD; John Iskander, MD, MPH; Sean V. Shadomy, DVM, MPH; Eric Weintraub, MPH; Robert T. Chen, MD, MA
JAMA. 2004;292:2478-2481.
Context An unexplained increase in the risk of Guillain-Barré syndrome (GBS) occurred among recipients of the swine influenza vaccine in 1976-1977. Guillain-Barré syndrome remains the most frequent neurological condition reported after influenza vaccination to the Vaccine Adverse Events Reporting System (VAERS) since its inception in 1990.
Objective To evaluate trends of reports to VAERS of GBS following influenza vaccination in adults.
Design, Setting, and Participants VAERS is the US national spontaneous reporting system for adverse events following vaccination. Reports of GBS in persons 18 years or older following influenza vaccination were evaluated for each influenza season from July 1, 1990, through June 30, 2003. The number of people vaccinated was estimated from the National Health Interview Survey and US census data. Beginning in 1994, active follow-up was conducted to verify GBS diagnosis and obtain other clinical details.
Main Outcome Measure Reporting rates of GBS following influenza vaccination over time.
Results From July 1990 through June 2003, VAERS received 501 reports of GBS following influenza vaccination in adults. The median onset interval (13 days) was longer than that of non-GBS reports of adverse events after influenza vaccine (1 day) (P<.001). The annual reporting rate decreased 4-fold from a high of 0.17 per 100 000 vaccinees in 1993-1994 to 0.04 in 2002-2003 (P<.001). A GBS diagnosis was confirmed in 82% of reports. Preceding illness within 4 weeks of vaccination was identified in 24% of reported cases.
Conclusions From 1990 to 2003, VAERS reporting rates of GBS after influenza vaccination decreased. The long onset interval and low prevalence of other preexisting illnesses are consistent with a possible causal association between GBS and influenza vaccine. These findings require additional research, which can lead to a fuller understanding of the causes of GBS and its possible relationship with influenza vaccine.
Author Affiliations: National Immunization Program, Centers for Disease Control and Prevention (Ms Haber, Drs DeStefano, Iskander, Shadomy, and Chen, and Mr Weintraub); and National Center for Infectious Diseases, Division of Bacterial and Mycotic Diseases (Dr Angulo), Atlanta, Ga.
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Legal Concerns and the Influenza Vaccine Shortage Mello and Brennan JAMA 2005;294:1817-1820.
Trends in Influenza Vaccination-Associated Guillain-Barre Syndrome Journal Watch Pediatrics and Adolescent Medicine 2005;2005:14-14.
Trends in Influenza Vaccination-Associated Guillain-Barre Syndrome Journal Watch Infectious Diseases 2004;2004:5-5.
Surround H1N1 Vaccine Which May Contain Thimerosal Concerns Surround H1N1 Vaccine Which May Contain Thimerosal July 30th, 2009 Concerns Surround H1N1 Vaccine Which May Contain Thimerosal
By Shaun Heasley
July 30, 2009A swine flu vaccine expected this fall is raising red flags because it will likely contain a preservative some link to autism despite scientific research repeatedly proving otherwise.
Government officials say they hope to have 160 million doses of a vaccine for swine flu, or H1N1, available by the fall when flu season is expected to kick in. A vaccine is currently going through clinical trials. Pregnant women, children and health care workers will be the first to be vaccinated.
Scientists say the swine flu vaccine will be similar to the flu vaccines provided annually with little fanfare. However, some consumer groups are reserved about the new vaccine because of the possibility that it could contain thimerosal in combination with a second additive in an effort to produce large quantities more quickly.
Furthermore, a vaccine offered to combat a different strain of the swine flu in the 1970s is linked to increased incidents of a neurological disorder.
But makers of the vaccine say they anticipate manufacturing two versions, one with and one without thimerosal, which ought to ease some fears, reports ABC News.
Read the Entire Story Here.
Copyright © 2009 Disability Scoop, LLC. All Rights Reserved.
HHS 2009 H1N1 Vaccine Development Activities
Fact Sheet
Overview The newly emergent 2009 H1N1 influenza virus is a novel virus with pandemic potential. Consistent with the National Strategy for Pandemic Influenza, HHS is committing funds for the production of pilot lots for clinical studies, as well as a bulk supply of antigen and adjuvant for use in a potential vaccine for the 2009 H1N1 which will become a part of the national stockpile of pre-pandemic influenza vaccines.
A vaccine is made from a virus or bacteria (referred to as an antigen), which causes the human body’s immune system to develop antibodies against a specific virus or bacteria so the body can recognize and fight the virus or bacteria. Adjuvants may be added to a vaccine to help generate a stronger immune response so less vaccine is needed for the body to recognize and fight a virus or bacteria.
Vaccine Development When the 2009 H1N1 strain was isolated and identified as a novel influenza virus, work began to prepare a virus reference strain. This is a standard practice when new influenza strains are discovered, where a clinical sample of the virus is mixed with another influenza virus that grows in eggs to develop a new virus that has some of the properties of the novel virus and the ability to grow in eggs. This work is necessary in order to create an influenza vaccine using conventional methods.
Once a virus reference strain is ready, it will be made available to influenza vaccine manufacturers in order to create a master virus seed, which prepares a virus to be used in making the vaccine.
HHS Contracting Activities Since 2004, HHS has contracted with manufacturers that currently hold U.S. licenses for flu vaccine as part of the National Strategy for Pandemic Influenza. In May 2009, HHS issued new orders on these contracts to produce a bulk supply of vaccine antigen and adjuvant and to produce pilot (also called investigational) lots of a 2009 H1N1 vaccine. Most will be stored in bulk, and a small amount will be prepared as vaccine for use in clinical studies to evaluate vaccine safety and the dosage required for a protective effect. This research will include studies with adjuvant to determine its safety and the effect it would have on the immune system’s response.
Orders for Bulk Supply of H1N1 Influenza Vaccine Antigen and Adjuvant: May 22, 2009