An MD wrote me and chided me for not posting this study which 'proves'
vaccines work. I have highlighted some areas in the study that I find
interesting. Take a look and tell me what you think.
Abstract
Background.
Concern about both safety and efficacy has made the use of whole-cell pertussis vaccines controversial. In some European countries, including Italy, the rate of vaccination against pertussis is low.
Methods.
We conducted a double-blind trial in Italy in which infants were randomly assigned to vaccination at two, four, and six months of age with an acellular Pertussis vaccine together with diphtheria and tetanus toxoids (DTP); a DTP vaccine containing whole-cell Pertussis (manufactured by Connaught Laboratories); or diphtheria and tetanus toxoids without pertussis (DT). The acellular DTP vaccine was either one containing filamentous hemagglutinin, pertactin, and pertussis toxin inactivated with formalin and glutaraldehyde (SmithKline Beecham) or one with filamentous hemagglutinin, pertactin, and genetically detoxified pertussis toxin (Chiron Biocine). Pertussis was defined as 21 days or more of paroxysmal cough, with infection confirmed by culture or serologic testing.
Results.
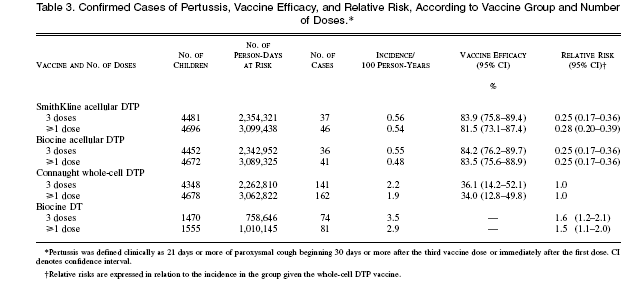
The efficacy of each vaccine, given in three doses, against pertussis was determined for 14,751 children over an average of 17 months, with cases included in the analysis if cough began 30 days or more after the completion of immunization. For both of the acellular DTP vaccines, the efficacy was 84 percent (95 percent confidence intervals, 76 to 89 percent for SmithKline DTP and 76 to 90 percent for Biocine DTP), whereas the efficacy of the whole-cell DTP vaccine was only 36 percent (95 percent confidence interval, 14 to 52 percent).
(If they worked wouldn't this be 100%?)
The antibody responses were greater to the acellular vaccines than to the whole-cell vaccine. Local and systemic adverse events were significantly more frequent
(adverse events? Like what? Seizures? Death?)
after the administration of the whole-cell vaccine. For the acellular vaccines, the frequency of adverse events was similar to that in the control (DT) group.
Conclusions.
The two acellular DTP vaccines we studied were safe, immunogenic, and efficacious against pertussis, whereas the efficacy of the whole-cell DTP vaccine was unexpectedly low.
( You mean all this time it hasn't really worked?)
(N Engl J Med 1996;334: 341-8.) In pediatrics the routine use of whole-cell vaccines against Bordetella pertussis has been a matter of continuous debate. 1-4 Acellular vaccines, consisting of purified proteins, have been in use for the primary immunization of two-year-old children in Japan since 1981. 5,6 Two acellular vaccines were evaluated in a randomized clinical trial in Sweden, 7 but the results left unanswered questions about the efficacy of the vaccines in infants, particularly in relation to that of whole-cell vaccines. In Italy, vaccination of infants against diphtheria, tetanus, poliomyelitis, and hepatitis B is mandatory, but vaccination against pertussis is not. The rate of vaccination against pertussis varies greatly according to year of birth and geographic area. 8 In 1991, the national average rate of vaccination against pertussis was estimated at 40 percent for children less than five years of age 9 ;this low level of coverage can be attributed to the perception that there is an unacceptably high frequency of adverse events after the administration of the wholecell vaccine. 8 In 1992, we initiated the present randomized, double-blind, controlled clinical trial of three Pertussis vaccines. The pertussis vaccines we studied were combined with diphtheria and tetanus toxoids and included a whole-cell vaccine currently used in the United States and two acellular vaccines, each containing inactive pertussis toxin, filamentous hemagglutinin, and pertactin. These proteins are involved in the pathogenesis of B. Pertussis infection, and animal studies have suggested that they confer active immunity. 10,11 Pertussis toxin has various biologic actions; because of its toxicity in animals, inactivation is required before it can beused as an immunogen. The main objective of the trial was to examine the efficacy of each vaccine, given at two, four, and six months of age, in preventing laboratory-confirmed clinical pertussis.
METHODS
Participants
Infants were enrolled from September 1992 to September 1993 at 62 public health clinics operated by the National Health System, located in 4 of the 20 regions of Italy: Piemonte, Veneto, Friuli–Venezia Giulia, and Puglia. Nurses were hired and trained specifically to enroll and follow study children and to record information on standardized forms and in customized computerized data bases. The inclusion criteria for the study are shown in Table 1. The parents of each eligible newborn were invited to enter the trial; those who agreed gave written, informed consent. The study was approved by the Italian National Committee for Bioethics and the institutional review board of the U.S. National Institute of Allergy and Infectious Diseases (NIAID).
Vaccination
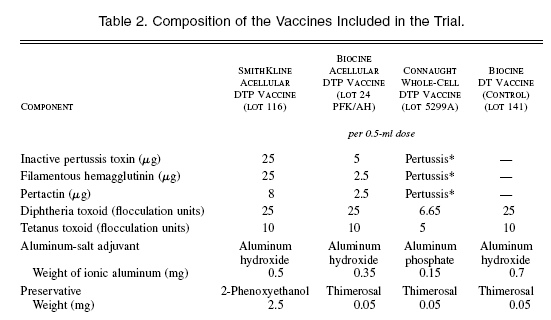
The composition of the study vaccines is shown in Table 2. The acellular diphtheria–tetanus–pertussis (DTP) vaccine manufactured by Chiron Biocine (Siena, Italy) contains genetically detoxified Pertussis toxin, 12 filamentous hemagglutinin, and pertactin. The acellular DTP vaccine manufactured by SmithKline Beecham Biologicals (Rixensart, Belgium) contains pertussis toxin inactivated with formalin and glutaraldehyde, filamentous hemagglutinin, and pertactin. The heat- inactivated whole-cell DTP manufactured by Connaught Laboratories (Swiftwater, Pa.) belonged to a commercial lot licensed in the United States; this same lot was used concurrently in Sweden in a randomized clinical trial of two other acellular vaccines. A commercial diphtheria– tetanus (DT) preparation (Biocine) was used for the control group. Three doses were administered when the infants were 6 to 12, 13 to 20, and 21 to 28 weeks of age, with a period of 4 to 12 weeks between successive doses. Criteria for discontinuing vaccination are included in Table 1. A booster dose of DT was given to all children six months after the third dose. The first two doses of the trial vaccine could be administered simultaneously with oral poliomyelitis vaccine and hepatitis B vaccine. The study vaccines were injected intramuscularly in the buttock or thigh.
Medical: Age 6–12 weeks
Weight: 3rd percentile for age
No history of seizures or other central nervous system disease, including perinatal brain damage
No major congenital abnormalities, failure to thrive, or renal failure
No known or suspected immunologic deficit, including having a
mother known to be positive for the human immunodeficiency virus
No prior illness compatible with pertussis
No prior vaccination against pertussis
Practical
Italian-speaking mother
Family accessible by telephone
Family planning to remain in the area for at least 12 months
CRITERIA CONTRAINDICATIONS TO FURTHER DOSES
Rectal temperature 40 C within 48 hours
Persistent crying for 3 hours within 48 hours
Hypotonic, hyporesponsive episode or collapse within 48 hours
Generalized cyanosis within 48 hours
Anaphylaxis within 24 hours
Seizure, encephalitis, encephalopathy, or other serious central nervous system disease at any time
*5.7 IU per dose by mouse intracerebral challenge test, as determined by the manufacturer.
Sample Size, Randomization, and Vaccine Masking
Assuming a true vaccine efficacy of 80 percent and a 5 percent incidence of laboratory-confirmed pertussis in unvaccinated children for the mean observation period, we calculated that a sample of 3300 children receiving each pertussis vaccine and 1100 receiving DT would provide an 85 percent probability that the lower limit of a two sided
95 percent confidence interval for vaccine efficacy would be greater than 60 percent. 13 Enrollment was augmented to compensate for attrition and potential overestimation of the incidence of pertussis. Ten sets of three doses each of vaccine were boxed together (three sets of each of the three DTP vaccines and one set of the DT vaccine, all in identical vials); the sets were consecutively numbered according to randomization lists provided by the NIAID. Infants were assigned to one of the four study groups at each clinic when they received the first dose of vaccine from the next available set of vials. Regular clinical personnel prepared and administered the vaccine. Neither parents nor investigators knew the infants’ vaccine assignments.
Data Collection and Analysis
Efficacy
Nurses performed active surveillance by means of routine monthly telephone calls. Parents were instructed to note each of the child’s episodes of coughing and report them to the study nurse. As soon as an illness characterized by cough lasting more than seven days was identified, samples of acute-phase serum and nasopharyngeal mucus were obtained; convalescent-phase serum was obtained six to eight weeks after the onset of cough. Parents recorded details of the illness and of treatment daily in a standardized diary; a nurse telephoned each week to review and record this information. The episode of coughing was considered over when the child had had no cough for two full days. Surveillance was conducted for coughing illnesses that began as late as December 31, 1994. Each child contributed time at risk for Pertussis through the date of onset of laboratory-confirmed disease, the last date of contact with a study nurse, or the end of the surveillance period. Vaccine efficacy was estimated for two periods, one beginning 30 days after the third dose of study vaccine and the other beginning immediately after the first dose. A case of pertussis was defined as an illness with 21 days or more of paroxysmal cough and evidence of B. Pertussis infection on culture or diagnostic serologic testing, as defined below. Vaccine efficacy after three doses was also estimated with the use of other clinical end points, according to the same laboratory criteria.
Abstract
Background.
Concern about both safety and efficacy has made the use of whole-cell pertussis vaccines controversial. In some European countries, including Italy, the rate of vaccination against pertussis is low.
Methods.
We conducted a double-blind trial in Italy in which infants were randomly assigned to vaccination at two, four, and six months of age with an acellular Pertussis vaccine together with diphtheria and tetanus toxoids (DTP); a DTP vaccine containing whole-cell Pertussis (manufactured by Connaught Laboratories); or diphtheria and tetanus toxoids without pertussis (DT). The acellular DTP vaccine was either one containing filamentous hemagglutinin, pertactin, and pertussis toxin inactivated with formalin and glutaraldehyde (SmithKline Beecham) or one with filamentous hemagglutinin, pertactin, and genetically detoxified pertussis toxin (Chiron Biocine). Pertussis was defined as 21 days or more of paroxysmal cough, with infection confirmed by culture or serologic testing.
Results.
The efficacy of each vaccine, given in three doses, against pertussis was determined for 14,751 children over an average of 17 months, with cases included in the analysis if cough began 30 days or more after the completion of immunization. For both of the acellular DTP vaccines, the efficacy was 84 percent (95 percent confidence intervals, 76 to 89 percent for SmithKline DTP and 76 to 90 percent for Biocine DTP), whereas the efficacy of the whole-cell DTP vaccine was only 36 percent (95 percent confidence interval, 14 to 52 percent).
(If they worked wouldn't this be 100%?)
The antibody responses were greater to the acellular vaccines than to the whole-cell vaccine. Local and systemic adverse events were significantly more frequent
(adverse events? Like what? Seizures? Death?)
after the administration of the whole-cell vaccine. For the acellular vaccines, the frequency of adverse events was similar to that in the control (DT) group.
Conclusions.
The two acellular DTP vaccines we studied were safe, immunogenic, and efficacious against pertussis, whereas the efficacy of the whole-cell DTP vaccine was unexpectedly low.
( You mean all this time it hasn't really worked?)
(N Engl J Med 1996;334: 341-8.) In pediatrics the routine use of whole-cell vaccines against Bordetella pertussis has been a matter of continuous debate. 1-4 Acellular vaccines, consisting of purified proteins, have been in use for the primary immunization of two-year-old children in Japan since 1981. 5,6 Two acellular vaccines were evaluated in a randomized clinical trial in Sweden, 7 but the results left unanswered questions about the efficacy of the vaccines in infants, particularly in relation to that of whole-cell vaccines. In Italy, vaccination of infants against diphtheria, tetanus, poliomyelitis, and hepatitis B is mandatory, but vaccination against pertussis is not. The rate of vaccination against pertussis varies greatly according to year of birth and geographic area. 8 In 1991, the national average rate of vaccination against pertussis was estimated at 40 percent for children less than five years of age 9 ;this low level of coverage can be attributed to the perception that there is an unacceptably high frequency of adverse events after the administration of the wholecell vaccine. 8 In 1992, we initiated the present randomized, double-blind, controlled clinical trial of three Pertussis vaccines. The pertussis vaccines we studied were combined with diphtheria and tetanus toxoids and included a whole-cell vaccine currently used in the United States and two acellular vaccines, each containing inactive pertussis toxin, filamentous hemagglutinin, and pertactin. These proteins are involved in the pathogenesis of B. Pertussis infection, and animal studies have suggested that they confer active immunity. 10,11 Pertussis toxin has various biologic actions; because of its toxicity in animals, inactivation is required before it can beused as an immunogen. The main objective of the trial was to examine the efficacy of each vaccine, given at two, four, and six months of age, in preventing laboratory-confirmed clinical pertussis.
METHODS
Participants
Infants were enrolled from September 1992 to September 1993 at 62 public health clinics operated by the National Health System, located in 4 of the 20 regions of Italy: Piemonte, Veneto, Friuli–Venezia Giulia, and Puglia. Nurses were hired and trained specifically to enroll and follow study children and to record information on standardized forms and in customized computerized data bases. The inclusion criteria for the study are shown in Table 1. The parents of each eligible newborn were invited to enter the trial; those who agreed gave written, informed consent. The study was approved by the Italian National Committee for Bioethics and the institutional review board of the U.S. National Institute of Allergy and Infectious Diseases (NIAID).
Vaccination
The composition of the study vaccines is shown in Table 2. The acellular diphtheria–tetanus–pertussis (DTP) vaccine manufactured by Chiron Biocine (Siena, Italy) contains genetically detoxified Pertussis toxin, 12 filamentous hemagglutinin, and pertactin. The acellular DTP vaccine manufactured by SmithKline Beecham Biologicals (Rixensart, Belgium) contains pertussis toxin inactivated with formalin and glutaraldehyde, filamentous hemagglutinin, and pertactin. The heat- inactivated whole-cell DTP manufactured by Connaught Laboratories (Swiftwater, Pa.) belonged to a commercial lot licensed in the United States; this same lot was used concurrently in Sweden in a randomized clinical trial of two other acellular vaccines. A commercial diphtheria– tetanus (DT) preparation (Biocine) was used for the control group. Three doses were administered when the infants were 6 to 12, 13 to 20, and 21 to 28 weeks of age, with a period of 4 to 12 weeks between successive doses. Criteria for discontinuing vaccination are included in Table 1. A booster dose of DT was given to all children six months after the third dose. The first two doses of the trial vaccine could be administered simultaneously with oral poliomyelitis vaccine and hepatitis B vaccine. The study vaccines were injected intramuscularly in the buttock or thigh.
Medical: Age 6–12 weeks
Weight: 3rd percentile for age
No history of seizures or other central nervous system disease, including perinatal brain damage
No major congenital abnormalities, failure to thrive, or renal failure
No known or suspected immunologic deficit, including having a
mother known to be positive for the human immunodeficiency virus
No prior illness compatible with pertussis
No prior vaccination against pertussis
Practical
Italian-speaking mother
Family accessible by telephone
Family planning to remain in the area for at least 12 months
CRITERIA CONTRAINDICATIONS TO FURTHER DOSES
Rectal temperature 40 C within 48 hours
Persistent crying for 3 hours within 48 hours
Hypotonic, hyporesponsive episode or collapse within 48 hours
Generalized cyanosis within 48 hours
Anaphylaxis within 24 hours
Seizure, encephalitis, encephalopathy, or other serious central nervous system disease at any time
*5.7 IU per dose by mouse intracerebral challenge test, as determined by the manufacturer.
Sample Size, Randomization, and Vaccine Masking
Assuming a true vaccine efficacy of 80 percent and a 5 percent incidence of laboratory-confirmed pertussis in unvaccinated children for the mean observation period, we calculated that a sample of 3300 children receiving each pertussis vaccine and 1100 receiving DT would provide an 85 percent probability that the lower limit of a two sided
95 percent confidence interval for vaccine efficacy would be greater than 60 percent. 13 Enrollment was augmented to compensate for attrition and potential overestimation of the incidence of pertussis. Ten sets of three doses each of vaccine were boxed together (three sets of each of the three DTP vaccines and one set of the DT vaccine, all in identical vials); the sets were consecutively numbered according to randomization lists provided by the NIAID. Infants were assigned to one of the four study groups at each clinic when they received the first dose of vaccine from the next available set of vials. Regular clinical personnel prepared and administered the vaccine. Neither parents nor investigators knew the infants’ vaccine assignments.
Data Collection and Analysis
Efficacy
Nurses performed active surveillance by means of routine monthly telephone calls. Parents were instructed to note each of the child’s episodes of coughing and report them to the study nurse. As soon as an illness characterized by cough lasting more than seven days was identified, samples of acute-phase serum and nasopharyngeal mucus were obtained; convalescent-phase serum was obtained six to eight weeks after the onset of cough. Parents recorded details of the illness and of treatment daily in a standardized diary; a nurse telephoned each week to review and record this information. The episode of coughing was considered over when the child had had no cough for two full days. Surveillance was conducted for coughing illnesses that began as late as December 31, 1994. Each child contributed time at risk for Pertussis through the date of onset of laboratory-confirmed disease, the last date of contact with a study nurse, or the end of the surveillance period. Vaccine efficacy was estimated for two periods, one beginning 30 days after the third dose of study vaccine and the other beginning immediately after the first dose. A case of pertussis was defined as an illness with 21 days or more of paroxysmal cough and evidence of B. Pertussis infection on culture or diagnostic serologic testing, as defined below. Vaccine efficacy after three doses was also estimated with the use of other clinical end points, according to the same laboratory criteria.
Immunogenicity
Paired capillary-blood samples were collected at each health center before the first dose of vaccine was given and one month after the third dose from a subsample of 10 percent of the children. Geometric mean titers of antibody to each pertussis antigen were measured before and after vaccination in each study group. A serologic response to each pertussis antigen was defined by a postvaccination titer at least four times higher than both the titer before vaccination and the minimal level of detection of the assay. Response to the diphtheria and tetanus toxoids was defined by a postvaccination antibody titer exceeding 0.01 IU per milliliter.
Safety
Parents recorded information on local and systemic symptoms in a standardized diary for the eight consecutive evenings after each vaccine dose was given. After the eighth day, nurses collected this information by telephone. If a serious illness occurred at any time, the study pediatricians verified the clinical history and reviewed all available documentation. Common side effects occurring during the two evenings after each dose were analyzed. Adverse events that were considered serious were anaphylaxis within 24 hours of vaccination; persistent crying (for 3 hours or more), a rectal temperature 40 C, hypotonic, hyporesponsive episodes, generalized cyanosis, or convulsions within 48 hours; and encephalopathy within 7 days.
Laboratory Procedures
Culture
Nasopharyngeal mucus was collected with an 8-French DeLee suction catheter (Sherwood Medical, St. Louis) and cultured on charcoal agar containing 10 percent defibrinated horse blood and 20 mg of cephalexin per liter (lot CM119, Unipath, Milan, Italy) in the regional laboratories. Isolates were identified by biochemical assay and by agglutination with specific antiserum (Murex Diagnostics, Dartford, England). All strains of bordetella were confirmed at the Istituto Superiore di Sanità in Rome.
Serologic Testing
Capillary blood was collected with Microtainer vials (Becton Dickinson, Rutherford, N. J.). All serologic assays were performed by personnel who had no knowledge of the infants’ vaccine assignments or the order of collection of the specimens; the samples were tested against pertussis antigens with reference serum calibrated against reference serum samples provided by the U.S. Food and Drug Administration (serum lot 3 or 4, Bethesda, Md.). Standardized enzyme-linked immunoassays (EIAs) were used to evaluate IgG and IgA antibodies to pertussis toxin and filamentous hemagglutinin and IgG antibodies to pertactin, 14 with antigens provided by SmithKline Beecham. The reference-line method was used to calculate EIA units with standardized software (Unitcalc, Biosys inova, Stockholm, 1992). The minimal level of detection was set at 2 units per milliliter for IgG antibody to pertussis toxin and filamentous hemagglutinin, 3 units per milliliter for both IgG antibody to pertactin and IgA antibody to filamentous hemagglutinin, and 10 units per milliliter for IgA antibody to Pertussis toxin. Any value below this minimum was recorded as half of the minimal value. The Chinese-hamster-ovary (CHO) assay of pertussis-toxin– neutralizing antibodies was performed only when sufficient serum was available. 15 Neutralization titers were expressed as the reciprocal of the highest serum dilution that caused complete inhibition of typical clustering; the minimal level of detection was the first dilution tested (1:40), and undetectable values were recorded as 1:20. For a serologic test to be considered positive, we required an increase in the antibody titer between acute-phase and convalescentphase serum samples that was equivalent to one of the following: (1) an increase in the level of IgG or IgA antibody to pertussis toxin to twice the initial value; (2) an increase in the level of IgG or IgA antibody to filamentous hemagglutinin to twice the initial value, provided culture and the polymerase-chain-reaction (PCR) assay were negative forB. parapertussis; or (3) an increase in the level of pertussis-toxin– neutralizing antibodies to four times the initial value. A diagnostic doubling of the value on the EIA required the convalescent-phase antibody level to be at least four times the minimal level of detection. Diphtheria antitoxin was assayed by toxin neutralization in Vero cells and tetanus antitoxin by a modified passive-hemagglutination assay 16,17 ; titers were converted to international units per milliliter with the use of an international reference serum.
B. parapertussis PCR
The PCR for the detection of B. parapertussis in aspirates involved the use of a specific insertion-sequence element IS1001.
18,19
Statistical Analysis
Vaccine efficacy was estimated as 1 _ R, where R is the ratio of the incidence of pertussis (ratio of cases to total person-time of follow-up) among the recipients of each DTP vaccine to the incidence among the control infants. 20 Confidence intervals were estimated by exact calculation, 21 on the basis of the conditional binomial distribution of cases in one vaccine group and the total number of cases.continuous variables were compared by means of the Kruskal–Wallis test. Differences between proportions were assessed by the chi-square test or Fisher’s exact test. No adjustment of P values was made for multiple comparisons. The Wilcoxon rank-sum test was used to compare mean log antibody titers.
RESULTS
A total of 15,601 infants (49.6 percent girls and 50.4 percent boys), making up about 25 percent of the eligible newborns, received the first study dose at a mean age of 10.5 weeks; 15,101 received the second dose (mean age, 17.8 weeks); and 14,832 received the third (mean age, 24.9 weeks). In each vaccine group, 89 percent of the doses were injected into the buttocks. At the time the first two trial doses were given, 92 percent of each vaccine group also received hepatitis B vaccine and 94 percent received oral poliomyelitis vaccine.22
Paired capillary-blood samples were collected at each health center before the first dose of vaccine was given and one month after the third dose from a subsample of 10 percent of the children. Geometric mean titers of antibody to each pertussis antigen were measured before and after vaccination in each study group. A serologic response to each pertussis antigen was defined by a postvaccination titer at least four times higher than both the titer before vaccination and the minimal level of detection of the assay. Response to the diphtheria and tetanus toxoids was defined by a postvaccination antibody titer exceeding 0.01 IU per milliliter.
Safety
Parents recorded information on local and systemic symptoms in a standardized diary for the eight consecutive evenings after each vaccine dose was given. After the eighth day, nurses collected this information by telephone. If a serious illness occurred at any time, the study pediatricians verified the clinical history and reviewed all available documentation. Common side effects occurring during the two evenings after each dose were analyzed. Adverse events that were considered serious were anaphylaxis within 24 hours of vaccination; persistent crying (for 3 hours or more), a rectal temperature 40 C, hypotonic, hyporesponsive episodes, generalized cyanosis, or convulsions within 48 hours; and encephalopathy within 7 days.
Laboratory Procedures
Culture
Nasopharyngeal mucus was collected with an 8-French DeLee suction catheter (Sherwood Medical, St. Louis) and cultured on charcoal agar containing 10 percent defibrinated horse blood and 20 mg of cephalexin per liter (lot CM119, Unipath, Milan, Italy) in the regional laboratories. Isolates were identified by biochemical assay and by agglutination with specific antiserum (Murex Diagnostics, Dartford, England). All strains of bordetella were confirmed at the Istituto Superiore di Sanità in Rome.
Serologic Testing
Capillary blood was collected with Microtainer vials (Becton Dickinson, Rutherford, N. J.). All serologic assays were performed by personnel who had no knowledge of the infants’ vaccine assignments or the order of collection of the specimens; the samples were tested against pertussis antigens with reference serum calibrated against reference serum samples provided by the U.S. Food and Drug Administration (serum lot 3 or 4, Bethesda, Md.). Standardized enzyme-linked immunoassays (EIAs) were used to evaluate IgG and IgA antibodies to pertussis toxin and filamentous hemagglutinin and IgG antibodies to pertactin, 14 with antigens provided by SmithKline Beecham. The reference-line method was used to calculate EIA units with standardized software (Unitcalc, Biosys inova, Stockholm, 1992). The minimal level of detection was set at 2 units per milliliter for IgG antibody to pertussis toxin and filamentous hemagglutinin, 3 units per milliliter for both IgG antibody to pertactin and IgA antibody to filamentous hemagglutinin, and 10 units per milliliter for IgA antibody to Pertussis toxin. Any value below this minimum was recorded as half of the minimal value. The Chinese-hamster-ovary (CHO) assay of pertussis-toxin– neutralizing antibodies was performed only when sufficient serum was available. 15 Neutralization titers were expressed as the reciprocal of the highest serum dilution that caused complete inhibition of typical clustering; the minimal level of detection was the first dilution tested (1:40), and undetectable values were recorded as 1:20. For a serologic test to be considered positive, we required an increase in the antibody titer between acute-phase and convalescentphase serum samples that was equivalent to one of the following: (1) an increase in the level of IgG or IgA antibody to pertussis toxin to twice the initial value; (2) an increase in the level of IgG or IgA antibody to filamentous hemagglutinin to twice the initial value, provided culture and the polymerase-chain-reaction (PCR) assay were negative forB. parapertussis; or (3) an increase in the level of pertussis-toxin– neutralizing antibodies to four times the initial value. A diagnostic doubling of the value on the EIA required the convalescent-phase antibody level to be at least four times the minimal level of detection. Diphtheria antitoxin was assayed by toxin neutralization in Vero cells and tetanus antitoxin by a modified passive-hemagglutination assay 16,17 ; titers were converted to international units per milliliter with the use of an international reference serum.
B. parapertussis PCR
The PCR for the detection of B. parapertussis in aspirates involved the use of a specific insertion-sequence element IS1001.
18,19
Statistical Analysis
Vaccine efficacy was estimated as 1 _ R, where R is the ratio of the incidence of pertussis (ratio of cases to total person-time of follow-up) among the recipients of each DTP vaccine to the incidence among the control infants. 20 Confidence intervals were estimated by exact calculation, 21 on the basis of the conditional binomial distribution of cases in one vaccine group and the total number of cases.continuous variables were compared by means of the Kruskal–Wallis test. Differences between proportions were assessed by the chi-square test or Fisher’s exact test. No adjustment of P values was made for multiple comparisons. The Wilcoxon rank-sum test was used to compare mean log antibody titers.
RESULTS
A total of 15,601 infants (49.6 percent girls and 50.4 percent boys), making up about 25 percent of the eligible newborns, received the first study dose at a mean age of 10.5 weeks; 15,101 received the second dose (mean age, 17.8 weeks); and 14,832 received the third (mean age, 24.9 weeks). In each vaccine group, 89 percent of the doses were injected into the buttocks. At the time the first two trial doses were given, 92 percent of each vaccine group also received hepatitis B vaccine and 94 percent received oral poliomyelitis vaccine.22
Efficacy
Of the 15,601 enrolled children, 769 did not receive three doses of study vaccine. The reported reasons for the failure to administer the three-dose series were similar among the study groups, except for withdrawals due to side effects of the vaccine, which were more frequent after receipt of the whole-cell DTP vaccine; 135 children were withdrawn because of side effects after receiving the whole-cell DTP vaccine, 14 the SmithKline acellular DTP vaccine, 17 the Biocine acellular DTP vaccine, and 6 the DT vaccine. Of the 14,832 children who received three doses, 19 received doses of different products, 3 received partial doses, and 59 were excluded from further observation within 29 days after the administration of the third dose (13 because they had laboratory- confirmed illness and 46 who were lost to followup); (well, you did inject it into them!) therefore, 14,751 children (94.6 percent of those who were randomly assigned to groups) were included in the analysis of the efficacy of three doses of vaccine. No significant differences were detected among these 14,751 children according to study group in terms of sex, age at vaccination, household size, number of children less than 13 years of age in the same household, or mean number of days of observation. The mean length of follow-up for these children was 523 days (17.2 months), beginning 30 days after the third dose of vaccine. A total of 5147 episodes of cough lasting more than seven days were reported, and biologic specimens were collected for 4942 (96.0 percent) of these episodes, after a median of eight days of cough. Of 474 episodes of cough that were confirmed by laboratory testing to be associated with B. pertussis, 288 were defined as cases (with 21 days or more of paroxysmal cough) (Table 3). The average age at the onset of pertussis in these 288 cases was 551 days (18.2 months). The only complication noted after pertussis was a seizure in a recipient of the DT vaccine, who recovered without sequelae. The proportion of cases confirmed by culture was 73 percent for the whole-cell DTP vaccine, 82 percent for theDT vaccine, 7 6 percent f or t he SmithKline a cellular DTP vaccine, and 67 percent for the Biocine acellular vaccine; the remaining cases were confirmed by serologic assays. Each of the two types of acellular vaccine was 84 percent efficacious after three doses, whereas the efficacy of the whole-cell DTP vaccine was 36 percent (Table 3). Estimates of vaccine efficacy based on only the 216 culture-confirmed cases were 85 percent for the SmithKline acellular DTP vaccine, 87 percent for the Biocine acellular vaccine, and 43 percent for the whole-cell DTP vaccine. Examination of alternative clinical criteria for confirmed B. pertussis infection indicates that the vaccine efficacy after three doses increased for illnesses with increasingly longer durations of cough, but it nonetheless remained low for the wholecell DTP vaccine (Table 4). For the entire group of 15,601 children randomly assigned to vaccine groups, biologic specimens were collected for 5152 episodes of cough over an average of 21.6 months of follow-up after the first dose. There were 531 episodes of cough laboratory-confirmed as associated with B. pertussis, of which 343 were cases with paroxysmal cough lasting 21 days or more (Table 3); for each vaccine, vaccine efficacy after the first dose was similar to that after three doses.
A total of 24 cases occurred from randomization through the 29 days after the third dose (8 in recipients f the SmithKline acellular vaccine, 1 in a recipient of the Biocine acellular vaccine, 12 in recipients of the whole-cell DTP vaccine, and 3 in recipients of the DT vaccine) — an insufficient number to permit meaningful estimates of the incremental efficacy of each dose. Another analysis was based on cumulative periods from 30 days after the first dose to either 29 days after the third dose or 231 days of age, if fewer than three doses were received (this corresponded to 29 days after the maximal age at which the third dose could be given). In this period, 5 cases occurred in recipients of the SmithKline acellular vaccine (for an incidence of 0.39 per 100 person-years), 1 in a recipient of the Biocine acellular vaccine (0.08 per 100 person-years), 10 in recipients of the whole-cell DTP vaccine (0.79 per 100 person-years), and 2 in recipients of the DT vaccine (0.48 per 100 person-years). In pairwise comparisons, the incidence in recipients of the Biocine acellular DTP vaccine differed significantly from that in recipients of the whole-cell DTP vaccine (exact P_0.006, assuming the binomial distribution).23
Immunogenicity
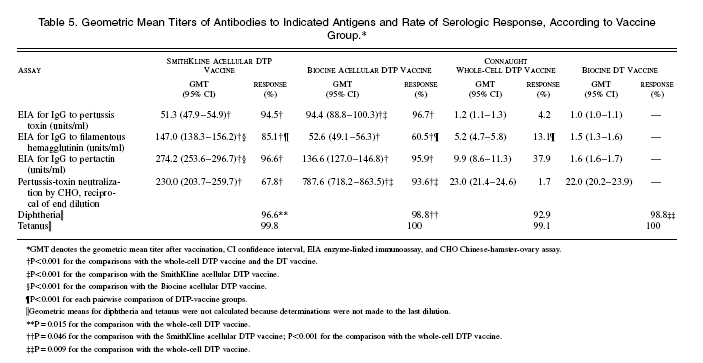
Serum specimens were obtained both before and after vaccination from 1572 children; 808 were tested by the CHO assay (Table 5). Antibody titers in the specimens obtained before immunization did not differ significantly among the groups. Each acellular vaccine elicited significantly higher titers of IgG and neutralizing antibody to pertussis toxin than did the whole-cell vaccine; the Biocine acellular DTP vaccine induced higher titers than the SmithKline acellular vaccine. In each group given an acellular DTP vaccine, the rate of serologic response on EIA was 94 percent or higher; the proportion with a serologic response by CHO assay was significantly higher for the Biocine acellular vaccine than for the SmithKline product. For the whole-cell DTP vaccine, the proportion of children with a serologic response to pertussis toxin by EIA or by CHO assay was minimal. Geometric mean titers of IgG antibody to filamentous hemagglutinin and pertactin were much higher after vaccination with the acellular DTP vaccine than with the whole-cell vaccine; in recipients of the SmithKline acellular DTP vaccine, titers were significantly higher than in recipients of the Biocine acellular DTP vaccine. The proportions of children with a serologic response to filamentous hemagglutinin differed significantly among the vaccine groups. For the two acellular DTP vaccines, the serologic response to pertactin was more than 95 percent, whereas it was significantly lower after the administration of the whole-cell DTP vaccine.
Safety
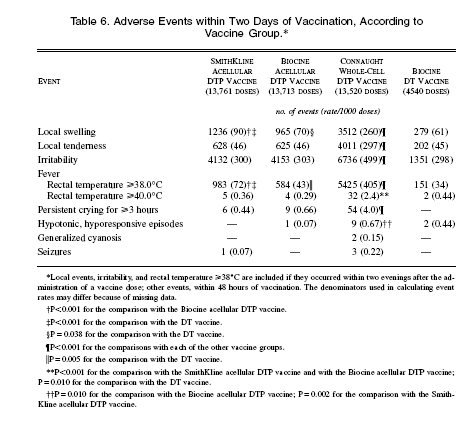
Table 6 shows the incidence of adverse events within the first two days (anything after two days is ignored?) after vaccination. The common events we investigated were significantly more frequent in the group given the whole-cell DTP vaccine; overall, the frequency of these events in the two groups given acellular DTP vaccine was similar to that in DT-vaccine recipients. Swelling was more frequently reported after the administration of the acellular DTP vaccine than after the administration of the DT vaccine; it was somewhat more frequent in the children who received Smith- Kline acellular DTP vaccine than in those who received the Biocine acellular vaccine. Rectal temperatures _38C were infrequent after vaccination with either of the acellular DTP vaccines but occurred significantly more often than in the DT-vaccine recipients; such temperatures were more frequent in recipients of the SmithKline vaccine than in recipients of the Biocine vaccine. In all but the recipients of the whole-cell DTP vaccine, the frequency of temperatures _38C and local symptoms increased with each dose in the series. Rectal temperature _40C, crying for three hours or more, and hypotonic, hyporesponsive episodes were rarely reported after the administration of the DT vaccine or the acellular DTP vaccines; (Rarely?) the frequency of these symptoms after the administration of the wholecell DTP vaccine was greater than in all the other groups. Although the frequency of temperatures _40C increased with each additional dose, persistent crying for three hours or more and hypotonic, hyporesponsive episodes were more frequently reported after the first dose. No episodes of anaphylaxis or encephalopathy were observed. All children who had severe events within 48 hours of vaccination recovered without sequelae. (Severe events?)
Of the 15,601 enrolled children, 769 did not receive three doses of study vaccine. The reported reasons for the failure to administer the three-dose series were similar among the study groups, except for withdrawals due to side effects of the vaccine, which were more frequent after receipt of the whole-cell DTP vaccine; 135 children were withdrawn because of side effects after receiving the whole-cell DTP vaccine, 14 the SmithKline acellular DTP vaccine, 17 the Biocine acellular DTP vaccine, and 6 the DT vaccine. Of the 14,832 children who received three doses, 19 received doses of different products, 3 received partial doses, and 59 were excluded from further observation within 29 days after the administration of the third dose (13 because they had laboratory- confirmed illness and 46 who were lost to followup); (well, you did inject it into them!) therefore, 14,751 children (94.6 percent of those who were randomly assigned to groups) were included in the analysis of the efficacy of three doses of vaccine. No significant differences were detected among these 14,751 children according to study group in terms of sex, age at vaccination, household size, number of children less than 13 years of age in the same household, or mean number of days of observation. The mean length of follow-up for these children was 523 days (17.2 months), beginning 30 days after the third dose of vaccine. A total of 5147 episodes of cough lasting more than seven days were reported, and biologic specimens were collected for 4942 (96.0 percent) of these episodes, after a median of eight days of cough. Of 474 episodes of cough that were confirmed by laboratory testing to be associated with B. pertussis, 288 were defined as cases (with 21 days or more of paroxysmal cough) (Table 3). The average age at the onset of pertussis in these 288 cases was 551 days (18.2 months). The only complication noted after pertussis was a seizure in a recipient of the DT vaccine, who recovered without sequelae. The proportion of cases confirmed by culture was 73 percent for the whole-cell DTP vaccine, 82 percent for theDT vaccine, 7 6 percent f or t he SmithKline a cellular DTP vaccine, and 67 percent for the Biocine acellular vaccine; the remaining cases were confirmed by serologic assays. Each of the two types of acellular vaccine was 84 percent efficacious after three doses, whereas the efficacy of the whole-cell DTP vaccine was 36 percent (Table 3). Estimates of vaccine efficacy based on only the 216 culture-confirmed cases were 85 percent for the SmithKline acellular DTP vaccine, 87 percent for the Biocine acellular vaccine, and 43 percent for the whole-cell DTP vaccine. Examination of alternative clinical criteria for confirmed B. pertussis infection indicates that the vaccine efficacy after three doses increased for illnesses with increasingly longer durations of cough, but it nonetheless remained low for the wholecell DTP vaccine (Table 4). For the entire group of 15,601 children randomly assigned to vaccine groups, biologic specimens were collected for 5152 episodes of cough over an average of 21.6 months of follow-up after the first dose. There were 531 episodes of cough laboratory-confirmed as associated with B. pertussis, of which 343 were cases with paroxysmal cough lasting 21 days or more (Table 3); for each vaccine, vaccine efficacy after the first dose was similar to that after three doses.
A total of 24 cases occurred from randomization through the 29 days after the third dose (8 in recipients f the SmithKline acellular vaccine, 1 in a recipient of the Biocine acellular vaccine, 12 in recipients of the whole-cell DTP vaccine, and 3 in recipients of the DT vaccine) — an insufficient number to permit meaningful estimates of the incremental efficacy of each dose. Another analysis was based on cumulative periods from 30 days after the first dose to either 29 days after the third dose or 231 days of age, if fewer than three doses were received (this corresponded to 29 days after the maximal age at which the third dose could be given). In this period, 5 cases occurred in recipients of the SmithKline acellular vaccine (for an incidence of 0.39 per 100 person-years), 1 in a recipient of the Biocine acellular vaccine (0.08 per 100 person-years), 10 in recipients of the whole-cell DTP vaccine (0.79 per 100 person-years), and 2 in recipients of the DT vaccine (0.48 per 100 person-years). In pairwise comparisons, the incidence in recipients of the Biocine acellular DTP vaccine differed significantly from that in recipients of the whole-cell DTP vaccine (exact P_0.006, assuming the binomial distribution).23
Immunogenicity
Serum specimens were obtained both before and after vaccination from 1572 children; 808 were tested by the CHO assay (Table 5). Antibody titers in the specimens obtained before immunization did not differ significantly among the groups. Each acellular vaccine elicited significantly higher titers of IgG and neutralizing antibody to pertussis toxin than did the whole-cell vaccine; the Biocine acellular DTP vaccine induced higher titers than the SmithKline acellular vaccine. In each group given an acellular DTP vaccine, the rate of serologic response on EIA was 94 percent or higher; the proportion with a serologic response by CHO assay was significantly higher for the Biocine acellular vaccine than for the SmithKline product. For the whole-cell DTP vaccine, the proportion of children with a serologic response to pertussis toxin by EIA or by CHO assay was minimal. Geometric mean titers of IgG antibody to filamentous hemagglutinin and pertactin were much higher after vaccination with the acellular DTP vaccine than with the whole-cell vaccine; in recipients of the SmithKline acellular DTP vaccine, titers were significantly higher than in recipients of the Biocine acellular DTP vaccine. The proportions of children with a serologic response to filamentous hemagglutinin differed significantly among the vaccine groups. For the two acellular DTP vaccines, the serologic response to pertactin was more than 95 percent, whereas it was significantly lower after the administration of the whole-cell DTP vaccine.
Safety
Table 6 shows the incidence of adverse events within the first two days (anything after two days is ignored?) after vaccination. The common events we investigated were significantly more frequent in the group given the whole-cell DTP vaccine; overall, the frequency of these events in the two groups given acellular DTP vaccine was similar to that in DT-vaccine recipients. Swelling was more frequently reported after the administration of the acellular DTP vaccine than after the administration of the DT vaccine; it was somewhat more frequent in the children who received Smith- Kline acellular DTP vaccine than in those who received the Biocine acellular vaccine. Rectal temperatures _38C were infrequent after vaccination with either of the acellular DTP vaccines but occurred significantly more often than in the DT-vaccine recipients; such temperatures were more frequent in recipients of the SmithKline vaccine than in recipients of the Biocine vaccine. In all but the recipients of the whole-cell DTP vaccine, the frequency of temperatures _38C and local symptoms increased with each dose in the series. Rectal temperature _40C, crying for three hours or more, and hypotonic, hyporesponsive episodes were rarely reported after the administration of the DT vaccine or the acellular DTP vaccines; (Rarely?) the frequency of these symptoms after the administration of the wholecell DTP vaccine was greater than in all the other groups. Although the frequency of temperatures _40C increased with each additional dose, persistent crying for three hours or more and hypotonic, hyporesponsive episodes were more frequently reported after the first dose. No episodes of anaphylaxis or encephalopathy were observed. All children who had severe events within 48 hours of vaccination recovered without sequelae. (Severe events?)
DISCUSSION
In this study, primary vaccination in infancy with either of two types of three-component acellular DTP vaccine was found to be highly effective in preventing clinical, laboratory-confirmed pertussis. Virtually all recipients of the DT vaccine who had confirmed disease had cough for 21 days or more (89 of 92 children), whereas the corresponding proportions in the recipients of the acellular DTP vaccines were lower (Smith- Kline, 58 of 84 children; Biocine, 63 of 82 children). Inclusion of a randomly assigned control group given only the DT vaccine provided precise estimates of absolute vaccine efficacy, without which estimates of efficacy in relation to that of the whole-cell vaccine could have been uninformative. The use of a U.S.- licensed whole-cell vaccine that was also included in the clinical trial conducted by the Swedish Institute for Infectious Disease Control24 provided a solid basis for interpreting the results of both trials. In the Swedish trial, the observed efficacy of the same whole-cell DTP vaccine was 48 percent (95 percent confidence interval, 37 to 58 percent); a five-component vaccine containing 10 mg of pertussis toxin inactivated with glutaraldehyde, 5 mg each of filamentous hemagglutinin and fimbrial antigens (serotypes 2 and 3), and 3mg of pertactin was highly efficacious (85 percent), whereas a two-component vaccine containing 25 mg each of filamentous hemagglutinin and pertussis toxin inactivated with formalin and glutaraldehyde had inferior efficacy (59 percent).24 In another placebo-controlled, randomized clinical trial in Sweden, 40 mg of peroxide- inactivated pertussis toxin administered at 3, 5, and 12 months of age was found to be 71 percent effective (95 percent confidence interval, 63 to 78 percent) in preventing laboratory-confirmed disease characterized by 21 days or more of paroxysmal coughing.25 The results of our trial are supported by a case–contact study of secondary transmission in households, in which the same three-component acellular DTP vaccine (SmithKline) was administered at two, four, and six months of age, with an estimated efficacy of 89 percent (95 percent confidence interval, 77 to 95 percent).26 Results of other studies of acellular vaccines are expected shortly.27,28 Different vaccine preparations, vaccination schedules, study designs, or determinations of outcome variables can limit the comparability of study results, however. The potential for bias exists in the laboratory confirmation of cases, since confirmation may be more limited in vaccinated children.29 However, with clinical Pertussis defined as 21 days or more of paroxysmal coughing, there appears to be no substantial bias; point estimates of vaccine efficacy were minimally altered when we considered only culture-confirmed cases. In the present trial, the efficacy of the two acellular DTP vaccines was similar despite differences in the methods of inactivating pertussis toxin and the amounts of the various antigens included in the vaccines. Although antibody responses to each acellular vaccine were high, differences in geometric mean antibody titers were observed between the two acellular vaccines. Antibody responses to the other antigens reflected the quantity of vaccine per dose, but titers of antibody to pertussis toxin did not correspond with the weight of antigens in each dose; this is consistent with other reports that the genetically inactivated pertussis toxin induces a stronger antibody response than chemically detoxified toxin.30 The weak antibody response to pertussis toxin and filamentous hemagglutinin induced by the Connaught wholecell DTP vaccine has been reported in other studies.31,32 The level of protection conferred by the whole-cell vaccine was lower than we anticipated. Caution must be used in interpreting differences in serologic responses to vaccines, however, since serologic responses to specific pertussis antigens have not been shown to be correlated with clinical protection.7 Despite this fact, and although responses were measured only to the antigens contained in the acellular DTP vaccines, these results suggest that the protective effectiveness of whole-cell vaccines should be questioned if their immunogenicity is low.33,34 Studies of whole-cell pertussis vaccines in general report a higher level of protective efficacy than we found, albeit with differences in methods and the possible influence of other factors, such as the administration of booster doses. 28,35,36 The frequencies of common and uncommon adverse events after the administration of the whole-cell DTP vaccine were similar to the rates previously reported for whole-cell vaccines and much higher than those observed in recipients of the acellular vaccines.37-39 There were only minimal differences between the two acellular DTP vaccines and the DT vaccine in this respect. Because of the high observed efficacy and the improved safety profile of the acellular DTP vaccines, their use for the immunization of infants appears highly preferable to the continued use of the whole-cell vaccines.
We are indebted to V. Rafti and his staff for their full engagement in the administrative management of the trial; to M. Kanieff for valuable assistance throughout the study; to the staff of the NIAID for technical support; to the members of the Steering Committee and the Data Safety Monitoring Committee, in particular to Professor G.M. Fara, who acted as safety officer; and to the staff members of the participating local health units, who actively contributed to the conduct
of the study.
APPENDIX
The members of the Progetto Pertosse Working Group in Italy were as follows: Laboratory of Bacteriology and Medical Mycology — P. Stefanelli, M. Bottone, and T. Sofia; Laboratory of Epidemiology and Biostatistics — S. Luzi, G. Bellomi, F. Cobianchi, G. Canganella, and F. Meduri; Laboratory of Immunology, Istituto Superiore di Sanità, Rome — G. Scuderi; Department of Hygiene and Microbiology, University of Palermo, Palermo — A. Chiarini, M. Maggio, S. Taormina, and M. Genovese; Piemonte region — A. Moiraghi, A. Barale, S. Di Tommaso, S. Malaspina, and E. Vasile; Veneto region — P. Ferraro, P. Dal Lago, L. De Marzi, L. Robino, and E. Giraldo; Friuli–Venezia Giulia region — N. Coppola, P. Materassi, G. Tarabini Castellani, and F. Basso; and Puglia region — S. Barbuti, M. Quarto, P. Lopalco, P. D’Orazio, and A. Sanguedolce.
REFERENCES
1. Kanai K. Japan’s experience in pertussis epidemiology and vaccination in
the past thirty years. Jpn J Med Sci Biol 1980;33:107-43.
2. Miller DL, Alderslade R, Ross EM. Whooping cough and whooping cough
vaccine: the risks and benefits debate. Epidemiol Rev 1982;4:1-24.
3. Romanus V, Jonsell R, Bergquist S-O. Pertussis in Sweden after the cessation
of general immunization in 1979. Pediatr Infect Dis J 1987;6:364-71.
4. Howson CP, Howe CJ, Fineberg HV. Adverse effects of pertussis and rubella
vaccines: a report of the Committee to Review the Adverse Consequences
of Pertussis and Rubella Vaccines. Washington, D.C.: National Academy
Press, 1991.
5. Kimura M, Kuno-Sakai H. Current epidemiology of pertussis in Japan. Pediatr
Infect Dis J 1990;9:705-9.
6. Mortimer EA Jr, Kimura M, Cherry JD, et al. Protective efficacy of the
Takeda acellular pertussis vaccine combined with diphtheria and tetanus
toxoids following household exposure of Japanese children. Am J Dis Child
1990;144:899-904.
7. Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial
of two acellular pertussis vaccines in Sweden — protective efficacy and adverse
events. Lancet 1988;1:955-60. [Erratum, Lancet 1988;1:1238.]
8. The Italian Vaccine Coverage Survey Working Group. Childhood vaccination
coverage in Italy: results of a seven-region survey. Bull World Health
Organ 1994;72:885-95.
9. Binkin NJ, Salmaso S, Tozzi AE, Scuderi G, Greco D. Epidemiology of pertussis
in a developed country with low vaccination coverage: the Italian experience.
Pediatr Infect Dis J 1992;11:653-61.
10. Oda M, Cowell JL, Burstyn DG, Manclark CR. Protective activities of the
filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella
pertussis in mice. J Infect Dis 1984;150:823-33.
11. Shahin RD, Brennan MJ, Li ZM, Meade BD, Manclark CR. Characterization
of the protective capacity and immunogenicity of the 69-kD outer
membrane protein of Bordetella pertussis. J Exp Med 1990;171:63-73.
12. Pizza M, Covacci A, Bartoloni A, et al. Mutants of pertussis toxin suitable
for vaccine development. Science 1989;246:497-500.
13. Blackwelder WC. Sample size and power for prospective analysis of relative
risk. Stat Med 1993;12:691-8.
14. Manclark CR, Meade BD, Burstyn DG. Serological response to Bordetella
pertussis. In: Rose NR, Friedman H, Fahey JL, eds. Manual of clinical laboratory
immunology. 3rd ed. Washington, D.C.: American Society for Microbiology,
1986:388-94.
15. Gillenius P, Jäätmaa E, Askelöf P, Granström M, Tiru M. The standardization
of an assay for pertussis toxin and antitoxin in microplate culture of
Chinese hamster ovary cells. J Biol Stand 1985;13:61-6.
16. Miyamura K, Tajiri E, Ito A, Murata R, Kono R. Micro cell culture method
for determination of diphtheria toxin and antitoxin titres using VERO cells.
II. Comparison with the rabbit skin method and practical application for seroepidemiological
studies. J Biol Stand 1974;2:203-9.
17. Pitzurra M, Bistoni F, Pitzurra L, Marconi B. Use of turkey red blood cells
in the passive haemagglutination test for studying tetanus immunity. Bull
World Health Organ 1983;61:331-8.
18. van der Zee A, Agterberg C, van Agterveld M, Peeters M, Mooi FR. Characterization
of IS1001, an insertion sequence element of Bordetella parapertussis.
J Bacteriol 1993;175:141-7.
19. van der Zee A, Agterberg C, Peeters M, Schellekens J, Mooi FR. Polymerase
chain reaction assay for pertussis: simultaneous detection and discrimination
of Bordetella pertussis and Bordetella parapertussis. J Clin Microbiol
1993;31:2134-40.
20. Breslow NE, Day NE. Statistical methods in cancer research. Vol. 2. The
design and analysis of cohort studies. Lyon, France: International Agency
for Research on Cancer, 1987. (IARC scientific publications no. 82.)
21. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated
in the case of the binomial. Biometrika 1934;26:404-13.
22. Johnson NL, Kotz S. Discrete distributions. Boston: Houghton Mifflin,
1969.
23. Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research: principles
and quantitative methods. Belmont, Calif.: Lifetime Learning, 1982.
24. Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled
trial of a two-component acellular, a five-component acellular, and
a whole-cell pertussis vaccine. N Engl J Med 1996;334:349-55.
25. Trollfors B, Taranger J, Lagergård T, et al. A placebo-controlled trial of a
pertussis-toxoid vaccine. N Engl J Med 1995;333:1045-50.
26. Schmitt H-J, Wirsing von König C-H, Neiss A, et al. Efficacy of acellular pertussis
vaccine in early childhood after household exposure. JAMA 1996;275:
37-41.
27. Edwards KM. Acellular pertussis vaccines — a solution to the pertussis
problem? J Infect Dis 1993;168:15-20.
28. Liese JG, Harzer E, Meschievitz CK, et al. Case-control study to evaluate
the efficacy of BIKEN acellular pertussis vaccine (Tripedia) combined with
diphtheria and tetanus toxoids (DTacP) in infants. In: Program and abstracts
of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy,
San Francisco, September 17–20, 1995. Washington, D.C.: American
Society for Microbiology, 1995:173. abstract.
348 THE NEW ENGLAND JOURNAL OF MEDICINE Feb. 8, 1996
29. Hallander HO, Storsaeter J, Möllby R. Evaluation of serology and nasopharyngeal
cultures for diagnosis of pertussis in a vaccine efficacy trial. J Infect
Dis 1991;163:1046-54.
30. Edwards KM, Meade BD, Decker MD, et al. Comparison of 13 acellular pertussis
vaccines: overview and serologic response. Pediatrics 1995;96:548-57.
31. Edwards KM, Decker MD, Halsey NA, et al. Differences in antibody response
to whole-cell pertussis vaccines. Pediatrics 1991;88:1019-23.
32. Kaplan SL, Lauer BA, Ward MA, et al. Immunogenicity and safety of Haemophilus
influenzae type b-tetanus protein conjugate vaccine alone or mixed
with diphtheria-tetanus-pertussis vaccine in infants. J Pediatr 1994;124:323-7.
33. Halperin SA, Bortolussi R, MacLean D, Chisholm N. Persistence of pertussis
in an immunized population: results of the Nova Scotia Enhanced Pertussis
Surveillance Program. J Pediatr 1989;115:686-93.
34. Baker JD, Halperin SA, Edwards K, Miller B, Decker M, Stephens D. Antibody
response to Bordetella pertussis antigens after immunization with
American and Canadian whole-cell vaccines. J Pediatr 1992;121:523-7.
35. Fine PEM, Clarkson JA. Reflections on the efficacy of pertussis vaccines.
Rev Infect Dis 1987;9:866-83.
36. Onorato IM, Wassilak SG, Meade B. Efficacy of whole-cell pertussis vaccine
in preschool children in the United States. JAMA 1992;267:2745-9.
37. Blennow M, Granström M, Jäätmaa E, Olin P. Primary immunization of infants
with an acellular pertussis vaccine in a double-blind randomized clinical
trial. Pediatrics 1988;82:293-9.
38. Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates
of adverse reactions associated with DTP and DT immunizations in infants
and children. Pediatrics 1981;68:650-60.
39. Decker MD, Edwards KM, Steinhoff MC, et al. Comparison of 13 acellular
pertussis vaccines: adverse reactions. Pediatrics 1995;96:557-66.
In this study, primary vaccination in infancy with either of two types of three-component acellular DTP vaccine was found to be highly effective in preventing clinical, laboratory-confirmed pertussis. Virtually all recipients of the DT vaccine who had confirmed disease had cough for 21 days or more (89 of 92 children), whereas the corresponding proportions in the recipients of the acellular DTP vaccines were lower (Smith- Kline, 58 of 84 children; Biocine, 63 of 82 children). Inclusion of a randomly assigned control group given only the DT vaccine provided precise estimates of absolute vaccine efficacy, without which estimates of efficacy in relation to that of the whole-cell vaccine could have been uninformative. The use of a U.S.- licensed whole-cell vaccine that was also included in the clinical trial conducted by the Swedish Institute for Infectious Disease Control24 provided a solid basis for interpreting the results of both trials. In the Swedish trial, the observed efficacy of the same whole-cell DTP vaccine was 48 percent (95 percent confidence interval, 37 to 58 percent); a five-component vaccine containing 10 mg of pertussis toxin inactivated with glutaraldehyde, 5 mg each of filamentous hemagglutinin and fimbrial antigens (serotypes 2 and 3), and 3mg of pertactin was highly efficacious (85 percent), whereas a two-component vaccine containing 25 mg each of filamentous hemagglutinin and pertussis toxin inactivated with formalin and glutaraldehyde had inferior efficacy (59 percent).24 In another placebo-controlled, randomized clinical trial in Sweden, 40 mg of peroxide- inactivated pertussis toxin administered at 3, 5, and 12 months of age was found to be 71 percent effective (95 percent confidence interval, 63 to 78 percent) in preventing laboratory-confirmed disease characterized by 21 days or more of paroxysmal coughing.25 The results of our trial are supported by a case–contact study of secondary transmission in households, in which the same three-component acellular DTP vaccine (SmithKline) was administered at two, four, and six months of age, with an estimated efficacy of 89 percent (95 percent confidence interval, 77 to 95 percent).26 Results of other studies of acellular vaccines are expected shortly.27,28 Different vaccine preparations, vaccination schedules, study designs, or determinations of outcome variables can limit the comparability of study results, however. The potential for bias exists in the laboratory confirmation of cases, since confirmation may be more limited in vaccinated children.29 However, with clinical Pertussis defined as 21 days or more of paroxysmal coughing, there appears to be no substantial bias; point estimates of vaccine efficacy were minimally altered when we considered only culture-confirmed cases. In the present trial, the efficacy of the two acellular DTP vaccines was similar despite differences in the methods of inactivating pertussis toxin and the amounts of the various antigens included in the vaccines. Although antibody responses to each acellular vaccine were high, differences in geometric mean antibody titers were observed between the two acellular vaccines. Antibody responses to the other antigens reflected the quantity of vaccine per dose, but titers of antibody to pertussis toxin did not correspond with the weight of antigens in each dose; this is consistent with other reports that the genetically inactivated pertussis toxin induces a stronger antibody response than chemically detoxified toxin.30 The weak antibody response to pertussis toxin and filamentous hemagglutinin induced by the Connaught wholecell DTP vaccine has been reported in other studies.31,32 The level of protection conferred by the whole-cell vaccine was lower than we anticipated. Caution must be used in interpreting differences in serologic responses to vaccines, however, since serologic responses to specific pertussis antigens have not been shown to be correlated with clinical protection.7 Despite this fact, and although responses were measured only to the antigens contained in the acellular DTP vaccines, these results suggest that the protective effectiveness of whole-cell vaccines should be questioned if their immunogenicity is low.33,34 Studies of whole-cell pertussis vaccines in general report a higher level of protective efficacy than we found, albeit with differences in methods and the possible influence of other factors, such as the administration of booster doses. 28,35,36 The frequencies of common and uncommon adverse events after the administration of the whole-cell DTP vaccine were similar to the rates previously reported for whole-cell vaccines and much higher than those observed in recipients of the acellular vaccines.37-39 There were only minimal differences between the two acellular DTP vaccines and the DT vaccine in this respect. Because of the high observed efficacy and the improved safety profile of the acellular DTP vaccines, their use for the immunization of infants appears highly preferable to the continued use of the whole-cell vaccines.
We are indebted to V. Rafti and his staff for their full engagement in the administrative management of the trial; to M. Kanieff for valuable assistance throughout the study; to the staff of the NIAID for technical support; to the members of the Steering Committee and the Data Safety Monitoring Committee, in particular to Professor G.M. Fara, who acted as safety officer; and to the staff members of the participating local health units, who actively contributed to the conduct
of the study.
APPENDIX
The members of the Progetto Pertosse Working Group in Italy were as follows: Laboratory of Bacteriology and Medical Mycology — P. Stefanelli, M. Bottone, and T. Sofia; Laboratory of Epidemiology and Biostatistics — S. Luzi, G. Bellomi, F. Cobianchi, G. Canganella, and F. Meduri; Laboratory of Immunology, Istituto Superiore di Sanità, Rome — G. Scuderi; Department of Hygiene and Microbiology, University of Palermo, Palermo — A. Chiarini, M. Maggio, S. Taormina, and M. Genovese; Piemonte region — A. Moiraghi, A. Barale, S. Di Tommaso, S. Malaspina, and E. Vasile; Veneto region — P. Ferraro, P. Dal Lago, L. De Marzi, L. Robino, and E. Giraldo; Friuli–Venezia Giulia region — N. Coppola, P. Materassi, G. Tarabini Castellani, and F. Basso; and Puglia region — S. Barbuti, M. Quarto, P. Lopalco, P. D’Orazio, and A. Sanguedolce.
REFERENCES
1. Kanai K. Japan’s experience in pertussis epidemiology and vaccination in
the past thirty years. Jpn J Med Sci Biol 1980;33:107-43.
2. Miller DL, Alderslade R, Ross EM. Whooping cough and whooping cough
vaccine: the risks and benefits debate. Epidemiol Rev 1982;4:1-24.
3. Romanus V, Jonsell R, Bergquist S-O. Pertussis in Sweden after the cessation
of general immunization in 1979. Pediatr Infect Dis J 1987;6:364-71.
4. Howson CP, Howe CJ, Fineberg HV. Adverse effects of pertussis and rubella
vaccines: a report of the Committee to Review the Adverse Consequences
of Pertussis and Rubella Vaccines. Washington, D.C.: National Academy
Press, 1991.
5. Kimura M, Kuno-Sakai H. Current epidemiology of pertussis in Japan. Pediatr
Infect Dis J 1990;9:705-9.
6. Mortimer EA Jr, Kimura M, Cherry JD, et al. Protective efficacy of the
Takeda acellular pertussis vaccine combined with diphtheria and tetanus
toxoids following household exposure of Japanese children. Am J Dis Child
1990;144:899-904.
7. Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial
of two acellular pertussis vaccines in Sweden — protective efficacy and adverse
events. Lancet 1988;1:955-60. [Erratum, Lancet 1988;1:1238.]
8. The Italian Vaccine Coverage Survey Working Group. Childhood vaccination
coverage in Italy: results of a seven-region survey. Bull World Health
Organ 1994;72:885-95.
9. Binkin NJ, Salmaso S, Tozzi AE, Scuderi G, Greco D. Epidemiology of pertussis
in a developed country with low vaccination coverage: the Italian experience.
Pediatr Infect Dis J 1992;11:653-61.
10. Oda M, Cowell JL, Burstyn DG, Manclark CR. Protective activities of the
filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella
pertussis in mice. J Infect Dis 1984;150:823-33.
11. Shahin RD, Brennan MJ, Li ZM, Meade BD, Manclark CR. Characterization
of the protective capacity and immunogenicity of the 69-kD outer
membrane protein of Bordetella pertussis. J Exp Med 1990;171:63-73.
12. Pizza M, Covacci A, Bartoloni A, et al. Mutants of pertussis toxin suitable
for vaccine development. Science 1989;246:497-500.
13. Blackwelder WC. Sample size and power for prospective analysis of relative
risk. Stat Med 1993;12:691-8.
14. Manclark CR, Meade BD, Burstyn DG. Serological response to Bordetella
pertussis. In: Rose NR, Friedman H, Fahey JL, eds. Manual of clinical laboratory
immunology. 3rd ed. Washington, D.C.: American Society for Microbiology,
1986:388-94.
15. Gillenius P, Jäätmaa E, Askelöf P, Granström M, Tiru M. The standardization
of an assay for pertussis toxin and antitoxin in microplate culture of
Chinese hamster ovary cells. J Biol Stand 1985;13:61-6.
16. Miyamura K, Tajiri E, Ito A, Murata R, Kono R. Micro cell culture method
for determination of diphtheria toxin and antitoxin titres using VERO cells.
II. Comparison with the rabbit skin method and practical application for seroepidemiological
studies. J Biol Stand 1974;2:203-9.
17. Pitzurra M, Bistoni F, Pitzurra L, Marconi B. Use of turkey red blood cells
in the passive haemagglutination test for studying tetanus immunity. Bull
World Health Organ 1983;61:331-8.
18. van der Zee A, Agterberg C, van Agterveld M, Peeters M, Mooi FR. Characterization
of IS1001, an insertion sequence element of Bordetella parapertussis.
J Bacteriol 1993;175:141-7.
19. van der Zee A, Agterberg C, Peeters M, Schellekens J, Mooi FR. Polymerase
chain reaction assay for pertussis: simultaneous detection and discrimination
of Bordetella pertussis and Bordetella parapertussis. J Clin Microbiol
1993;31:2134-40.
20. Breslow NE, Day NE. Statistical methods in cancer research. Vol. 2. The
design and analysis of cohort studies. Lyon, France: International Agency
for Research on Cancer, 1987. (IARC scientific publications no. 82.)
21. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated
in the case of the binomial. Biometrika 1934;26:404-13.
22. Johnson NL, Kotz S. Discrete distributions. Boston: Houghton Mifflin,
1969.
23. Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research: principles
and quantitative methods. Belmont, Calif.: Lifetime Learning, 1982.
24. Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled
trial of a two-component acellular, a five-component acellular, and
a whole-cell pertussis vaccine. N Engl J Med 1996;334:349-55.
25. Trollfors B, Taranger J, Lagergård T, et al. A placebo-controlled trial of a
pertussis-toxoid vaccine. N Engl J Med 1995;333:1045-50.
26. Schmitt H-J, Wirsing von König C-H, Neiss A, et al. Efficacy of acellular pertussis
vaccine in early childhood after household exposure. JAMA 1996;275:
37-41.
27. Edwards KM. Acellular pertussis vaccines — a solution to the pertussis
problem? J Infect Dis 1993;168:15-20.
28. Liese JG, Harzer E, Meschievitz CK, et al. Case-control study to evaluate
the efficacy of BIKEN acellular pertussis vaccine (Tripedia) combined with
diphtheria and tetanus toxoids (DTacP) in infants. In: Program and abstracts
of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy,
San Francisco, September 17–20, 1995. Washington, D.C.: American
Society for Microbiology, 1995:173. abstract.
348 THE NEW ENGLAND JOURNAL OF MEDICINE Feb. 8, 1996
29. Hallander HO, Storsaeter J, Möllby R. Evaluation of serology and nasopharyngeal
cultures for diagnosis of pertussis in a vaccine efficacy trial. J Infect
Dis 1991;163:1046-54.
30. Edwards KM, Meade BD, Decker MD, et al. Comparison of 13 acellular pertussis
vaccines: overview and serologic response. Pediatrics 1995;96:548-57.
31. Edwards KM, Decker MD, Halsey NA, et al. Differences in antibody response
to whole-cell pertussis vaccines. Pediatrics 1991;88:1019-23.
32. Kaplan SL, Lauer BA, Ward MA, et al. Immunogenicity and safety of Haemophilus
influenzae type b-tetanus protein conjugate vaccine alone or mixed
with diphtheria-tetanus-pertussis vaccine in infants. J Pediatr 1994;124:323-7.
33. Halperin SA, Bortolussi R, MacLean D, Chisholm N. Persistence of pertussis
in an immunized population: results of the Nova Scotia Enhanced Pertussis
Surveillance Program. J Pediatr 1989;115:686-93.
34. Baker JD, Halperin SA, Edwards K, Miller B, Decker M, Stephens D. Antibody
response to Bordetella pertussis antigens after immunization with
American and Canadian whole-cell vaccines. J Pediatr 1992;121:523-7.
35. Fine PEM, Clarkson JA. Reflections on the efficacy of pertussis vaccines.
Rev Infect Dis 1987;9:866-83.
36. Onorato IM, Wassilak SG, Meade B. Efficacy of whole-cell pertussis vaccine
in preschool children in the United States. JAMA 1992;267:2745-9.
37. Blennow M, Granström M, Jäätmaa E, Olin P. Primary immunization of infants
with an acellular pertussis vaccine in a double-blind randomized clinical
trial. Pediatrics 1988;82:293-9.
38. Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates
of adverse reactions associated with DTP and DT immunizations in infants
and children. Pediatrics 1981;68:650-60.
39. Decker MD, Edwards KM, Steinhoff MC, et al. Comparison of 13 acellular
pertussis vaccines: adverse reactions. Pediatrics 1995;96:557-66.