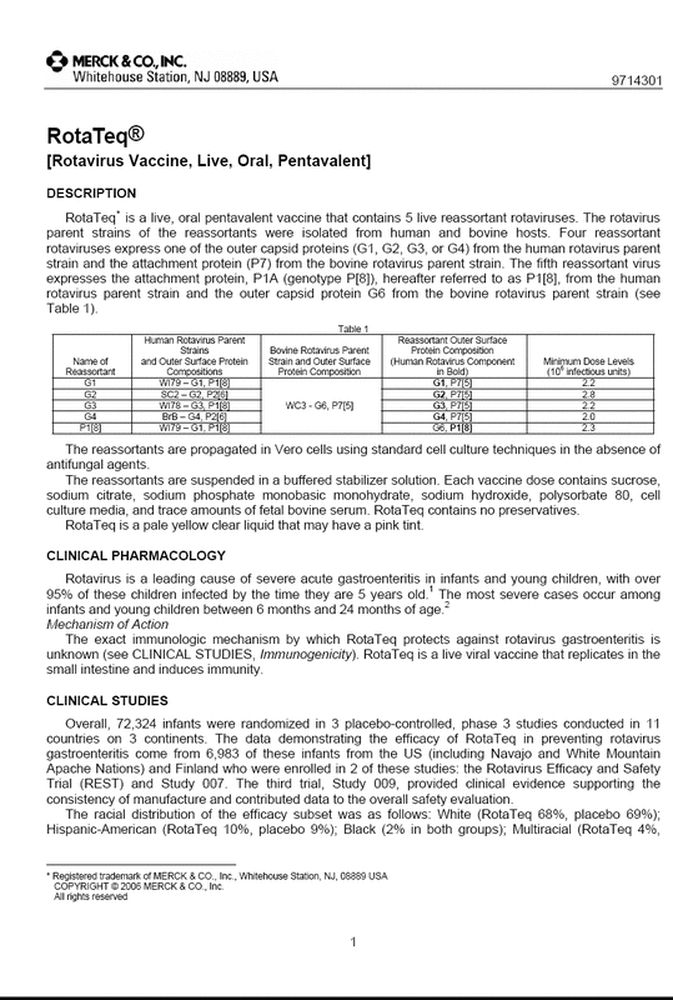
Examples of shedding vaccines:
J Infect Dis. 2004 Jul 15;190(2):409-16. Epub 2004 Jun 18. Related Articles,
Links
Shedding of Sabin Poliovirus Type 3 Containing the Nucleotide 472 Uracil-to-Cytosine Point Mutation after Administration of Oral Poliovirus Vaccine.
Martinez CV, Old MO, Kwock DK, Khan SS, Garcia JJ, Chan CS, Webster R, Falkovitz-Halpern MS, Maldonado YA.
Department of Pediatrics, Stanford University School of Medicine, Stanford, California, USA.
A uracil-to-cytosine point mutation at nucleotide (nt) 472 of Sabin oral poliovirus vaccine (OPV) type 3 is found in conjunction with vaccine-associated paralytic poliomyelitis (VAPP). Direct RNA extraction and mutant analysis by polymerase chain reaction and restriction enzyme cleavage were used to identify this point mutation in clinical samples. A total of 238 stool samples were obtained from 28 healthy infants for 6 weeks after OPV vaccination. More than 25% of infants shed OPV3 in the week after vaccination, with a decrease on day 6.
A second wave of OPV3 shedding occurred beginning the second week after vaccination and was maintained through the end of the study period. During the first week after vaccination, the proportion of nt 472 mutants in the shed OPV3 increased from undetectable to almost 100%. During the second shedding period, the proportion of nt 472 mutants remained close to 100%. These results suggest that selective mutation drives the VAPP-associated nt 472 point mutation for
OPV3 in the human gastrointestinal tract.
PMID: 15216480 [PubMed - in process]
J Virol. 2005 Jan;79(2):1062-70. Related Articles, Links
Spread of vaccine-derived poliovirus from a paralytic case in an immunodeficient child: an insight into the natural evolution of oral polio vaccine.
Cherkasova EA, Yakovenko ML, Rezapkin GV, Korotkova EA, Ivanova OE, Eremeeva TP, Krasnoproshina LI, Romanenkova NI, Rozaeva NR, Sirota L, Agol VI, Chumakov KM.
Center for Biologics Evaluation and Research, Food and Drug Administration, 1401 Rockville Pike, HFM-470, Rockville, MD 20852-1448. chumakov@cber.fda.gov.
Sabin strains used in the manufacture of oral polio vaccine (OPV) replicate in the human organism and can give rise to vaccine-derived polioviruses. The increased neurovirulence of vaccine derivatives has been known since the beginning of OPV use, but their ability to establish circulation in communities has been recognized only recently during the latest stages of the polio eradication campaign. This important observation called for studies of their emergence and evolution as well as extensive surveillance to determine the scope of this phenomenon. Here, we present the results of a study of vaccine-derived isolates from an immunocompromised poliomyelitis patient, the contacts, and the local sewage. All isolates were identified as closely related and slightly evolved vaccine derivatives with a recombinant type 2/type 1 genome. The strains also shared several amino acid substitutions including a mutation in the VP1 protein that was previously shown to be associated with the loss of attenuation. Another mutation in the VP3 protein resulted in altered immunological properties of the isolates, possibly facilitating virus spread in immunized populations. The patterns and rates of the accumulation of synonymous mutations in isolates collected from the patient over the extended period of excretion suggest either a substantially nonuniform rate of mutagenesis throughout the genome, or, more likely, the strains may have been intratypic recombinants between coevolving derivatives with different degrees of divergence from the vaccine parent. This study provides insight into the early stages of the establishment of circulation by runaway vaccine strains.
PMID: 15613335 [PubMed - in process]
Parents ‘need to be aware of specific immunisations’
By a staff reporter
1 September 2005
DUBAI — As immunisations are essential in the healthy growth of children, Belhoul Speciality Hospital's Paediatrician Dr Ashok Lodha says parents should be aware of the specifics, myths and effects especially as children are heading back to school.
As separate diseases and infections exist in different parts of the globe, the World Health Organisation (WHO) recommends immunisation protocols depending on epidermiological and geographical factors of each country.
Dr Lodha says that as the majority of people living in the UAE are expatriates, it is essential that parents are aware of the specific immunisations for this region.
Parents are often unaware of when to administer vaccine Dr Lodha advised vaccine such as Bacille Calmette-Guerin (BCG) given at birth, Hepatitis B at birth, two, four and six months, Diphtheria Pertussis, Haemophilius Influenza (Hib) at two, four, six and 15 months, Oral Polio (OPV) at two, four, six and 18 months, and Measles Mumps and Rubella (MMR) at one year and booster at five to six years.
There are some myths, which develop about the effects of some vaccinations, but generally they are false, says Dr Lodha.
"Some thought that there was a connection between MMR and autism but this is just media hype and it is a scientifically proven fact that the only similarity is the age of onset as signs of autism occur around the age of vaccine," he said.
While most controversial vaccine carry no scientifically proven effects, there are some vaccine that do. One such vaccine is known as the 5-vaccine involving hepatitis A, hepatitis B, pertussi, diphtheria and tetanus given in one single prick.
"The 5-Vaccine contains thimerosal, a mercury preservative which was banned by the Food and Drug Administration in over-the-counter drug preparations because of its safety," said Dr Lodha. "It contains aluminum which accumulates in the brain, muscle and bone tissue. As 5-vaccine is developed from animal ingredients it can also be linked to contracting viruses. Also 5-vaccine is a live virus meaning other people in close contact with the recipient can become infected, especially pregnant women."
http://hsc.virginia.edu/med-ed/micro/vir/vir4.html
B. Live-attenuated vaccines
Live attenuated vaccines are produced by extensive serial passage of the pathogenic virus in cell culture or animals. As the virus is passaged it loses its virulence for the human host due to the accumulation of mutations in virus genes. Although its virulence is lost, the virus retains its ability to replicate in the human host and to provoke an immune response that is effective in controlling the pathogenic strain. Live-attenuated vaccine strains are created under conditions in which multiple mutations accumulate in the virus genome. The presence of multiple mutations minimizes the possibility that the vaccine strain will revert to the pathogenic form. It is often the case, however, that investigators have not identified the exact nucleotide changes that lead to loss of virus virulence.
Since live-attenuated vaccine strains replicate in the human host, virus antigens are presented by MHC class I molecules and a strong cell-mediated response is induced. An anti-viral antibody response is also provoked. However, since a cell-mediated response is often central to controlling virus infections, the ability to induce a cellular response is an important part of the reason live-attenuated vaccines can be particularly effective. Compared to their killed vaccine counterparts, live-attenuated vaccines generally induce a longer lasting immunity. Live-attenuated vaccines are available for protection against measles, mumps, rubella, chicken pox and other diseases.
The ability of live-attenuated vaccine strains to replicate in the host causes particular concerns that do not arise with killed vaccines. For instance, reversion of the live-attenuated vaccine strain to wild type is a possibility. Since a reverted vaccine can cause disease, reversion is a concern even if the rate is very low. The case of the Sabin, live-attenuated polio vaccine illustrates this point. This vaccine was withdrawn from use even though it causes disease by way of reversion in only one in 4 million people vaccinated. Since paralytic polio is now so rare, this rate of reversion is considered too great for the vaccine to be in general use.
A second reservation regarding the use of live-attenuated vaccines has to do with the possibility that the vaccine strain may produce disease in the immunosuppressed. Live-attenuated vaccines are not deliberately given to immunosuppressed patients. Such patients may acquire them, however, from vaccinated individuals who are shedding the attenuated vaccine strain. Family members and other contacts are at risk. The danger of transmission to the immunosuppressed lasts for as long as the vaccine strain is replicated in the host.
http://rgfn.epcc.edu/rgfn/vacgl.htm
Live vaccines -- are constructed from the living organism (usually a virus). This is an attenuated strain; that is, it has been weakened in some fashion such that it will not cause (or more accurately should not cause) disease. The organism will however, cause the individual to generate an immune response and thus be immune to the wild (virulent - disease causing) form of the disease. There are certain inherent problems and benefits from live vaccines. First, because they are live, you can catch (although usually a milder form) the disease you are being vaccinated against; this side effect is not common. An advantage of the live vaccine is that the vaccinated person can, and often does, shed the vaccine organism (that is; spread it to others) thus others not directly vaccinated may also acquire the vaccine and become immune. The down side of this shedding is that a nonimmune person (or the infant in a nonimmune mother) can also become ill from the vaccine acquired by this indirect route. Therefore, special considerations must be applied when the vaccinated person may be exposed to nonimmmue or immunocompromised persons.
http://www.sh.lsuhsc.edu/new_curric/mod2/Micro_Infectious_Diseases
/2002%20%20RUBELLA%20Lecture%20.doc.
10). CONTROL--VACCINE: LIVE, ATTENUATED virus vaccine Give SC; Strain RA27/3 in human WI-38 cells. Required for school attendance. Usually, given with measles and mumps as "MMR" vaccine. Give first at l5 months to infants; Never give vaccine to pregnant woman or to female of child-bearing age unless she agrees not to become pregnant for 3 months. Vaccine is safe and induces good immunity. Medical personnel are a big factor in spreading rubella. Vaccine prevents viremia and significant shedding after reinfection. Vaccinees may develop viremia and may shed virus for 3 weeks from URT. Risk of vaccine virus to cause defects of fetus in pregnant women is considered negligible and NOT a reason to terminate a pregnancy. Vaccine virus is excreted in breast milk after postpartum immunization, but this is not risk for the infant. The RA/27/3 Rubella Vaccine is safe.
http://www.twincities.com/mld/pioneerpress
/12795127.htm?template=contentModules/printstory.jsp
Posted on Sun, Oct. 02, 2005
Infant found to have polio
Risk to general public is nil, state health officials say
BY TIM HUBER
Pioneer Press
Minnesota health officials said Saturday they are investigating the first case of polio reported in the state since 2000, but dismissed the potential for an outbreak of the crippling disease.
The case involves an infant from central Minnesota who already was hospitalized with a weakened immune system when the infection was discovered, the Minnesota Health Department said. The agency stressed that there is no risk to the general public. Only people who have not been vaccinated and who had direct contact with the infant would be at risk because the disease is transmitted through stools or oral secretions, said Health Department spokesman Buddy Ferguson. The vast majority of Minnesotans — approximately 93 percent — have been vaccinated, most as infants.
"If you didn't have that kind of contact, you're not at risk," Ferguson said. "We're talking about health care workers who might have cared for the infant. We're talking about family members. We are going to be contacting people in those groups individually."
In the vast majority of cases, polio has no symptoms, but it can cause sore throats, vomiting, abdominal pain and flulike symptoms, according to the Centers for Disease Control. In less than 2 percent of cases, the virus causes minor stiffness in the neck, back or legs, and in less than 1 percent of cases, it attacks the central nervous system and results in permanent paralysis, muscular atrophy and even death. The disease usually affects young children.
Before the 1960s, the disease left hundreds of thousands of victims paralyzed across the United States. Outbreaks created panic. Many people became afraid of large public gatherings. In 1946, the Minnesota State Fair was canceled as state health officials were recording as many as 50 new cases a day.
Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said the most recent case has been managed well by state officials.
"This is not a public health situation of any concern," he said.
Health officials would not name the child, give an age or even which town or hospital the infant is in, citing state and federal privacy laws.
The infant apparently contracted polio from someone infected with a mutated form of the polio strain used in oral vaccines, officials said. Even though the child is infected with the virus, he did not show any signs of paralysis, health officials said.
The United States stopped using polio vaccines that contain weakened strains of the live virus in 2000. The live-virus vaccine still can cause polio, and about eight people in the U.S. developed the disease annually before the country switched to using vaccine without the live virus. The last reported polio case in the U.S. occurred the final year that the live vaccine was in use.
Live-virus vaccine remains in use elsewhere in the world, in part because it helps expand protection in areas where not everyone receives the vaccine, Osterholm said. In essence, health officials rely on poor hygiene and inadequate sanitation in these areas to expose more people to the weakened form of the virus and thus give them protection.
At times, the weakened virus reverts to an infectious strain, which apparently is what happened to the infant in Minnesota, Osterholm said.
Polio ceased to be a major threat in the United States decades ago and largely has been eliminated in the Western Hemisphere since Dr. Jonas Salk developed the first vaccine in 1955. Yet the disease remains a serious health threat in the developing world and retains a terrifying reputation in the United States. By one estimate, polio paralyzed 254,000 Americans, many of them children. Vaccination gradually eliminated U.S. outbreaks. In 1960, there were 2,525 paralytic polio cases in the country, but by 1965 the number had dropped to 61, according to the Centers for Disease Control in Atlanta.
The last U.S. outbreak — and naturally occurring cases — occurred in 1979, when Amish in several Midwestern states contracted the disease from a strain of the virus brought in from the Netherlands, according to the CDC.
The last reported case of polio in Minnesota was caused by a vaccine in 1992.
In parts of Africa and Asia, however, polio remains a threat. In Indonesia, for instance, more than 230 children younger than 5 have been infected this year.
The Associated Press contributed to this report.
Tim Huber can be reached at thuber@pioneerpress.com or 651-228-5580.
http://www.startribune.com/stories/462/5649776.html
Minneapolis Star Tribune (subscription), MN
Minnesota's polio case is a health puzzler
Maura Lerner, Star Tribune
October 4, 2005
How did a baby in central Minnesota contract the virus that causes polio, a crippling disease that was essentially wiped out in the United States a quarter century ago?
That question has mystified state and federal health officials since tests confirmed the polio virus in an unidentified infant last week. The case is especially puzzling because the baby, who was born in this country, was somehow exposed to a strain of virus found in oral polio vaccines, which haven't been used in the United States for five years.
"[It] is not a public health concern for the general public," said Kris Ehresmann, chief of immunization at the Minnesota Health Department. "But it is definitely a situation that is of great scientific interest. It's a unique situation." Investigators now are testing relatives and others who have had close contact with the child to see whether anyone else may have been infected. They suspect that someone contracted the polio virus in another country and unwittingly passed it on.
The baby had no symptoms of polio, Ehresmann said. The virus was discovered during tests while the child was hospitalized for an unrelated immune condition. Officials declined to identify the child's gender or age, saying only that he or she is less than a year old. The Health Department was asked to run lab tests to find out whether a virus was making the child sick. When no routine viruses showed up, they started looking for obscure ones. And they found the polio virus.
The child hadn't been vaccinated against polio, apparently because of underlying medical problems. But health experts were astonished at the test results, to put it mildly. It's been 50 years since the polio vaccine was developed, in the midst of an epidemic that paralyzed as many as 21,000 Americans a year at its peak. By 1979, the disease had been wiped out as a natural threat in the United States.
For the next 20 years, virtually the only cases reported in this country -- an average of eight a year -- were caused by the oral vaccine, which used a modified live virus. Five years ago, the U.S. discontinued the oral vaccine and now uses a shot made from a killed virus that doesn't cause illness. Since then, federal officials say, no one had contracted polio in the United States.
To make sure of their findings, state officials sent samples of the virus to the U.S. Centers for Disease Control and Prevention in Atlanta for more tests. Last week, the agency confirmed that it's polio. "It's an unusual thing in any country," said Dr. Jim Alexander, a vaccine specialist at the federal agency. "There are many more questions so far than we have answers."
But they learned something remarkable, Ehresmann said. Using genetic fingerprinting, the CDC experts discovered that the virus strain had been used in an oral vaccine two years ago. That means that someone got the oral vaccine elsewhere -- it's still used in much of the world --and inadvertently transmitted the polio virus to someone else.
"You could have somebody who ... would appear completely healthy who could be unknowingly shedding virus," she said. It is transmitted by direct contact with stool (i.e. diapers). Typically, she said, people can only infect others for about a week. But people with immune problems may harbor it indefinitely. The baby is still hospitalized.
"I really hope that we'll be able to figure things out," Ehresmann said. "But it certainly is a possibility that there will still be some missing pieces to this puzzle when all is said and done."
Maura Lerner is at mlerner@startribune.com.
J Infect Dis. 2004 Jul 15;190(2):409-16. Epub 2004 Jun 18. Related Articles,
Links
Shedding of Sabin Poliovirus Type 3 Containing the Nucleotide 472 Uracil-to-Cytosine Point Mutation after Administration of Oral Poliovirus Vaccine.
Martinez CV, Old MO, Kwock DK, Khan SS, Garcia JJ, Chan CS, Webster R, Falkovitz-Halpern MS, Maldonado YA.
Department of Pediatrics, Stanford University School of Medicine, Stanford, California, USA.
A uracil-to-cytosine point mutation at nucleotide (nt) 472 of Sabin oral poliovirus vaccine (OPV) type 3 is found in conjunction with vaccine-associated paralytic poliomyelitis (VAPP). Direct RNA extraction and mutant analysis by polymerase chain reaction and restriction enzyme cleavage were used to identify this point mutation in clinical samples. A total of 238 stool samples were obtained from 28 healthy infants for 6 weeks after OPV vaccination. More than 25% of infants shed OPV3 in the week after vaccination, with a decrease on day 6.
A second wave of OPV3 shedding occurred beginning the second week after vaccination and was maintained through the end of the study period. During the first week after vaccination, the proportion of nt 472 mutants in the shed OPV3 increased from undetectable to almost 100%. During the second shedding period, the proportion of nt 472 mutants remained close to 100%. These results suggest that selective mutation drives the VAPP-associated nt 472 point mutation for
OPV3 in the human gastrointestinal tract.
PMID: 15216480 [PubMed - in process]
J Virol. 2005 Jan;79(2):1062-70. Related Articles, Links
Spread of vaccine-derived poliovirus from a paralytic case in an immunodeficient child: an insight into the natural evolution of oral polio vaccine.
Cherkasova EA, Yakovenko ML, Rezapkin GV, Korotkova EA, Ivanova OE, Eremeeva TP, Krasnoproshina LI, Romanenkova NI, Rozaeva NR, Sirota L, Agol VI, Chumakov KM.
Center for Biologics Evaluation and Research, Food and Drug Administration, 1401 Rockville Pike, HFM-470, Rockville, MD 20852-1448. chumakov@cber.fda.gov.
Sabin strains used in the manufacture of oral polio vaccine (OPV) replicate in the human organism and can give rise to vaccine-derived polioviruses. The increased neurovirulence of vaccine derivatives has been known since the beginning of OPV use, but their ability to establish circulation in communities has been recognized only recently during the latest stages of the polio eradication campaign. This important observation called for studies of their emergence and evolution as well as extensive surveillance to determine the scope of this phenomenon. Here, we present the results of a study of vaccine-derived isolates from an immunocompromised poliomyelitis patient, the contacts, and the local sewage. All isolates were identified as closely related and slightly evolved vaccine derivatives with a recombinant type 2/type 1 genome. The strains also shared several amino acid substitutions including a mutation in the VP1 protein that was previously shown to be associated with the loss of attenuation. Another mutation in the VP3 protein resulted in altered immunological properties of the isolates, possibly facilitating virus spread in immunized populations. The patterns and rates of the accumulation of synonymous mutations in isolates collected from the patient over the extended period of excretion suggest either a substantially nonuniform rate of mutagenesis throughout the genome, or, more likely, the strains may have been intratypic recombinants between coevolving derivatives with different degrees of divergence from the vaccine parent. This study provides insight into the early stages of the establishment of circulation by runaway vaccine strains.
PMID: 15613335 [PubMed - in process]
Parents ‘need to be aware of specific immunisations’
By a staff reporter
1 September 2005
DUBAI — As immunisations are essential in the healthy growth of children, Belhoul Speciality Hospital's Paediatrician Dr Ashok Lodha says parents should be aware of the specifics, myths and effects especially as children are heading back to school.
As separate diseases and infections exist in different parts of the globe, the World Health Organisation (WHO) recommends immunisation protocols depending on epidermiological and geographical factors of each country.
Dr Lodha says that as the majority of people living in the UAE are expatriates, it is essential that parents are aware of the specific immunisations for this region.
Parents are often unaware of when to administer vaccine Dr Lodha advised vaccine such as Bacille Calmette-Guerin (BCG) given at birth, Hepatitis B at birth, two, four and six months, Diphtheria Pertussis, Haemophilius Influenza (Hib) at two, four, six and 15 months, Oral Polio (OPV) at two, four, six and 18 months, and Measles Mumps and Rubella (MMR) at one year and booster at five to six years.
There are some myths, which develop about the effects of some vaccinations, but generally they are false, says Dr Lodha.
"Some thought that there was a connection between MMR and autism but this is just media hype and it is a scientifically proven fact that the only similarity is the age of onset as signs of autism occur around the age of vaccine," he said.
While most controversial vaccine carry no scientifically proven effects, there are some vaccine that do. One such vaccine is known as the 5-vaccine involving hepatitis A, hepatitis B, pertussi, diphtheria and tetanus given in one single prick.
"The 5-Vaccine contains thimerosal, a mercury preservative which was banned by the Food and Drug Administration in over-the-counter drug preparations because of its safety," said Dr Lodha. "It contains aluminum which accumulates in the brain, muscle and bone tissue. As 5-vaccine is developed from animal ingredients it can also be linked to contracting viruses. Also 5-vaccine is a live virus meaning other people in close contact with the recipient can become infected, especially pregnant women."
http://hsc.virginia.edu/med-ed/micro/vir/vir4.html
B. Live-attenuated vaccines
Live attenuated vaccines are produced by extensive serial passage of the pathogenic virus in cell culture or animals. As the virus is passaged it loses its virulence for the human host due to the accumulation of mutations in virus genes. Although its virulence is lost, the virus retains its ability to replicate in the human host and to provoke an immune response that is effective in controlling the pathogenic strain. Live-attenuated vaccine strains are created under conditions in which multiple mutations accumulate in the virus genome. The presence of multiple mutations minimizes the possibility that the vaccine strain will revert to the pathogenic form. It is often the case, however, that investigators have not identified the exact nucleotide changes that lead to loss of virus virulence.
Since live-attenuated vaccine strains replicate in the human host, virus antigens are presented by MHC class I molecules and a strong cell-mediated response is induced. An anti-viral antibody response is also provoked. However, since a cell-mediated response is often central to controlling virus infections, the ability to induce a cellular response is an important part of the reason live-attenuated vaccines can be particularly effective. Compared to their killed vaccine counterparts, live-attenuated vaccines generally induce a longer lasting immunity. Live-attenuated vaccines are available for protection against measles, mumps, rubella, chicken pox and other diseases.
The ability of live-attenuated vaccine strains to replicate in the host causes particular concerns that do not arise with killed vaccines. For instance, reversion of the live-attenuated vaccine strain to wild type is a possibility. Since a reverted vaccine can cause disease, reversion is a concern even if the rate is very low. The case of the Sabin, live-attenuated polio vaccine illustrates this point. This vaccine was withdrawn from use even though it causes disease by way of reversion in only one in 4 million people vaccinated. Since paralytic polio is now so rare, this rate of reversion is considered too great for the vaccine to be in general use.
A second reservation regarding the use of live-attenuated vaccines has to do with the possibility that the vaccine strain may produce disease in the immunosuppressed. Live-attenuated vaccines are not deliberately given to immunosuppressed patients. Such patients may acquire them, however, from vaccinated individuals who are shedding the attenuated vaccine strain. Family members and other contacts are at risk. The danger of transmission to the immunosuppressed lasts for as long as the vaccine strain is replicated in the host.
http://rgfn.epcc.edu/rgfn/vacgl.htm
Live vaccines -- are constructed from the living organism (usually a virus). This is an attenuated strain; that is, it has been weakened in some fashion such that it will not cause (or more accurately should not cause) disease. The organism will however, cause the individual to generate an immune response and thus be immune to the wild (virulent - disease causing) form of the disease. There are certain inherent problems and benefits from live vaccines. First, because they are live, you can catch (although usually a milder form) the disease you are being vaccinated against; this side effect is not common. An advantage of the live vaccine is that the vaccinated person can, and often does, shed the vaccine organism (that is; spread it to others) thus others not directly vaccinated may also acquire the vaccine and become immune. The down side of this shedding is that a nonimmune person (or the infant in a nonimmune mother) can also become ill from the vaccine acquired by this indirect route. Therefore, special considerations must be applied when the vaccinated person may be exposed to nonimmmue or immunocompromised persons.
http://www.sh.lsuhsc.edu/new_curric/mod2/Micro_Infectious_Diseases
/2002%20%20RUBELLA%20Lecture%20.doc.
10). CONTROL--VACCINE: LIVE, ATTENUATED virus vaccine Give SC; Strain RA27/3 in human WI-38 cells. Required for school attendance. Usually, given with measles and mumps as "MMR" vaccine. Give first at l5 months to infants; Never give vaccine to pregnant woman or to female of child-bearing age unless she agrees not to become pregnant for 3 months. Vaccine is safe and induces good immunity. Medical personnel are a big factor in spreading rubella. Vaccine prevents viremia and significant shedding after reinfection. Vaccinees may develop viremia and may shed virus for 3 weeks from URT. Risk of vaccine virus to cause defects of fetus in pregnant women is considered negligible and NOT a reason to terminate a pregnancy. Vaccine virus is excreted in breast milk after postpartum immunization, but this is not risk for the infant. The RA/27/3 Rubella Vaccine is safe.
http://www.twincities.com/mld/pioneerpress
/12795127.htm?template=contentModules/printstory.jsp
Posted on Sun, Oct. 02, 2005
Infant found to have polio
Risk to general public is nil, state health officials say
BY TIM HUBER
Pioneer Press
Minnesota health officials said Saturday they are investigating the first case of polio reported in the state since 2000, but dismissed the potential for an outbreak of the crippling disease.
The case involves an infant from central Minnesota who already was hospitalized with a weakened immune system when the infection was discovered, the Minnesota Health Department said. The agency stressed that there is no risk to the general public. Only people who have not been vaccinated and who had direct contact with the infant would be at risk because the disease is transmitted through stools or oral secretions, said Health Department spokesman Buddy Ferguson. The vast majority of Minnesotans — approximately 93 percent — have been vaccinated, most as infants.
"If you didn't have that kind of contact, you're not at risk," Ferguson said. "We're talking about health care workers who might have cared for the infant. We're talking about family members. We are going to be contacting people in those groups individually."
In the vast majority of cases, polio has no symptoms, but it can cause sore throats, vomiting, abdominal pain and flulike symptoms, according to the Centers for Disease Control. In less than 2 percent of cases, the virus causes minor stiffness in the neck, back or legs, and in less than 1 percent of cases, it attacks the central nervous system and results in permanent paralysis, muscular atrophy and even death. The disease usually affects young children.
Before the 1960s, the disease left hundreds of thousands of victims paralyzed across the United States. Outbreaks created panic. Many people became afraid of large public gatherings. In 1946, the Minnesota State Fair was canceled as state health officials were recording as many as 50 new cases a day.
Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said the most recent case has been managed well by state officials.
"This is not a public health situation of any concern," he said.
Health officials would not name the child, give an age or even which town or hospital the infant is in, citing state and federal privacy laws.
The infant apparently contracted polio from someone infected with a mutated form of the polio strain used in oral vaccines, officials said. Even though the child is infected with the virus, he did not show any signs of paralysis, health officials said.
The United States stopped using polio vaccines that contain weakened strains of the live virus in 2000. The live-virus vaccine still can cause polio, and about eight people in the U.S. developed the disease annually before the country switched to using vaccine without the live virus. The last reported polio case in the U.S. occurred the final year that the live vaccine was in use.
Live-virus vaccine remains in use elsewhere in the world, in part because it helps expand protection in areas where not everyone receives the vaccine, Osterholm said. In essence, health officials rely on poor hygiene and inadequate sanitation in these areas to expose more people to the weakened form of the virus and thus give them protection.
At times, the weakened virus reverts to an infectious strain, which apparently is what happened to the infant in Minnesota, Osterholm said.
Polio ceased to be a major threat in the United States decades ago and largely has been eliminated in the Western Hemisphere since Dr. Jonas Salk developed the first vaccine in 1955. Yet the disease remains a serious health threat in the developing world and retains a terrifying reputation in the United States. By one estimate, polio paralyzed 254,000 Americans, many of them children. Vaccination gradually eliminated U.S. outbreaks. In 1960, there were 2,525 paralytic polio cases in the country, but by 1965 the number had dropped to 61, according to the Centers for Disease Control in Atlanta.
The last U.S. outbreak — and naturally occurring cases — occurred in 1979, when Amish in several Midwestern states contracted the disease from a strain of the virus brought in from the Netherlands, according to the CDC.
The last reported case of polio in Minnesota was caused by a vaccine in 1992.
In parts of Africa and Asia, however, polio remains a threat. In Indonesia, for instance, more than 230 children younger than 5 have been infected this year.
The Associated Press contributed to this report.
Tim Huber can be reached at thuber@pioneerpress.com or 651-228-5580.
http://www.startribune.com/stories/462/5649776.html
Minneapolis Star Tribune (subscription), MN
Minnesota's polio case is a health puzzler
Maura Lerner, Star Tribune
October 4, 2005
How did a baby in central Minnesota contract the virus that causes polio, a crippling disease that was essentially wiped out in the United States a quarter century ago?
That question has mystified state and federal health officials since tests confirmed the polio virus in an unidentified infant last week. The case is especially puzzling because the baby, who was born in this country, was somehow exposed to a strain of virus found in oral polio vaccines, which haven't been used in the United States for five years.
"[It] is not a public health concern for the general public," said Kris Ehresmann, chief of immunization at the Minnesota Health Department. "But it is definitely a situation that is of great scientific interest. It's a unique situation." Investigators now are testing relatives and others who have had close contact with the child to see whether anyone else may have been infected. They suspect that someone contracted the polio virus in another country and unwittingly passed it on.
The baby had no symptoms of polio, Ehresmann said. The virus was discovered during tests while the child was hospitalized for an unrelated immune condition. Officials declined to identify the child's gender or age, saying only that he or she is less than a year old. The Health Department was asked to run lab tests to find out whether a virus was making the child sick. When no routine viruses showed up, they started looking for obscure ones. And they found the polio virus.
The child hadn't been vaccinated against polio, apparently because of underlying medical problems. But health experts were astonished at the test results, to put it mildly. It's been 50 years since the polio vaccine was developed, in the midst of an epidemic that paralyzed as many as 21,000 Americans a year at its peak. By 1979, the disease had been wiped out as a natural threat in the United States.
For the next 20 years, virtually the only cases reported in this country -- an average of eight a year -- were caused by the oral vaccine, which used a modified live virus. Five years ago, the U.S. discontinued the oral vaccine and now uses a shot made from a killed virus that doesn't cause illness. Since then, federal officials say, no one had contracted polio in the United States.
To make sure of their findings, state officials sent samples of the virus to the U.S. Centers for Disease Control and Prevention in Atlanta for more tests. Last week, the agency confirmed that it's polio. "It's an unusual thing in any country," said Dr. Jim Alexander, a vaccine specialist at the federal agency. "There are many more questions so far than we have answers."
But they learned something remarkable, Ehresmann said. Using genetic fingerprinting, the CDC experts discovered that the virus strain had been used in an oral vaccine two years ago. That means that someone got the oral vaccine elsewhere -- it's still used in much of the world --and inadvertently transmitted the polio virus to someone else.
"You could have somebody who ... would appear completely healthy who could be unknowingly shedding virus," she said. It is transmitted by direct contact with stool (i.e. diapers). Typically, she said, people can only infect others for about a week. But people with immune problems may harbor it indefinitely. The baby is still hospitalized.
"I really hope that we'll be able to figure things out," Ehresmann said. "But it certainly is a possibility that there will still be some missing pieces to this puzzle when all is said and done."
Maura Lerner is at mlerner@startribune.com.
CDC reports sexual transmission of vaccinia May 4, 2007 (CIDRAP News) – A woman from Alaska experienced vulvar vaccinia after she was intimate with a US military member who had received his smallpox vaccination 3 days before, according to a report from the US Centers for Disease Control and Prevention (CDC).
The report, published in today's issue of Morbidity and Mortality Weekly Report (MMWR), said the woman sought treatment at a public health clinic in Alaska in October 2006 for increasingly painful vaginal tears that she said were not caused by sexual abuse or trauma.
Vaccinia, a rare cutaneous and sometimes systemic reaction to smallpox vaccination that can occur in vaccinees or in close contacts of vaccinees, has been reported more frequently in the medical literature since the US military revived its long-discontinued smallpox vaccination program in late 2002. By June 2006 about 1.1 million US service members bound for the Middle East and other areas thought to be high-risk had been vaccinated.
In March a 2-year-old Indiana boy was hospitalized with severe eczema vaccinatum that he contracted from his father, a soldier who had recently received a smallpox shot.
In the woman's case, in early October 2006 clinicians swabbed one of her two vulvar lesions and diagnosed secondary candidiasis; however, her symptoms continued to worsen despite treatment with over-the-counter medication, the MMWR report said. A healthcare provider then diagnosed cellulitis, discontinued the over-the-counter remedy, and prescribed a 7-day course of oral cephalexin. The patient's symptoms resolved 1 week later.
In the meanwhile, the Alaska State Virology Laboratory (ASVL) found the woman did not have herpes simplex virus, but it was unable to identify what virus she had. About a month later, the virus remained unidentified after the ASVL submitted it to a reference lab.
On Jan 9, 2007, the ASVL submitted the unidentified isolate to the CDC, where cloning and sequencing of her sample, based on products from specialized polymerase chain reaction (PCR) testing, identified DNA bands that matched vaccinia virus sequences. Additional PCR testing performed by the CDC Poxvirus Laboratory revealed that the woman's isolate was consistent with a vaccine-strain vaccinia virus, and the lab relayed the results to the ASVL on Jan 30.
Upon receiving the woman's test result, Alaska state health officials interviewed the woman and learned that she had never been vaccinated against smallpox but that the only sex partner she had had from 1 month before her infection until the lesions were healed was a US service member. She said their intimate contact involved manual stimulation and vaginal intercourse, and she didn't remember seeing bandages or unusual skin lesions on her partner.
When state health officials interviewed medical officers at the military base, they found that the soldier had been deployed overseas in late October. He had received a smallpox vaccination on Sep 19, 2006, along with instruction on vaccination site care and proper hand hygiene. They found no other transmission of the virus from either the soldier or from the woman to other people, including the heathcare workers who examined her.
The US military revived its long-discontinued smallpox vaccination program in late 2002, and by June 2006 about 1.1 million service members had been vaccinated.
Contact vaccinia, as in the woman's case, is more common than the more severe eczema vaccinatum or progressive vaccinia. According to the US Department of Defense (DoD) Web site, 61 cases (36 lab-confirmed) of contact vaccinia occurred, mainly to spouses and adult intimate contacts, between Dec 13, 2002, and Apr 12, 2007. The MMWR report said lab-confirmed vulvar vaccinia after sexual contact with vaccinated military members has also been reported in New York and Texas since the DoD resumed its smallpox vaccination program.
The CDC and the DoD have received four reports of nongenital contact vaccinia since Mar 8, 2007, related to recently vaccinated service members, the MMWR report said.
In an editorial note that accompanied the report of the woman's case, the CDC wrote that to prevent contact vaccinia transfers, healthcare providers should educate vaccinees on proper handwashing after bandage changesas well as on how to avoid other contact with the vaccination site. It said asking patients who seek treatment for vesicular lesions that resemble vaccinia about contact with recent smallpox vaccines can speed diagnosis and provide quicker contact tracing, clinical intervention, and counseling about preventing further transmission.
CDC. Vulvar vaccinia infection after sexual contact with a military smallpox vaccine—Alaska, 2006. MMWR 2007 May 4;56(17):417-19 [Full text]
(doi:10.1542/peds.2009-1901)
Published online January 25, 2010
Case Reports
Sibling Transmission of Vaccine-Derived Rotavirus (RotaTeq) Associated With Rotavirus Gastroenteritis
Daniel C. Payne, PhD, MSPHa, Kathryn M. Edwards, MDb, Michael D. Bowen, PhDc, Erin Keckley, RNb, Jody Peters, MSb, Mathew D. Esona, PhDc, Elizabeth N. Teel, BSc, Diane Kent, RNb, Umesh D. Parashar, MBBS, MPHa, Jon R. Gentsch, PhDc
Although rotavirus vaccines are known to be shed in stools, transmission of vaccine-derived virus to unvaccinated contacts resulting in symptomatic rotavirus gastroenteritis has not been reported to our knowledge. We document here the occurrence of vaccine-derived rotavirus (RotaTeq [Merck and Co, Whitehouse Station, NJ]) transmission from a vaccinated infant to an older, unvaccinated sibling, resulting in symptomatic rotavirus gastroenteritis that required emergency department care. Results of our investigation suggest that reassortment between vaccine component strains of genotypes P7[5]G1 and P1A[8]G6 occurred during replication either in the vaccinated infant or in the older sibling, raising the possibility that this reassortment may have increased the virulence of the vaccine-derived virus. Both children remain healthy 11 months after this event and are without underlying medical conditions.
Key Words: rotavirus • acute gastroenteritis • RotaTeq • rotavirus vaccine • WC3 • sibling transmission • horizontal transmission • shedding • reassortant • New Vaccine Surveillance Network • NVSN
Abbreviations: RT-PCR, reverse transcription-polymerase chain reaction
Accepted Nov 20, 2009.
(15) MMWR, Recommendations and Reports, April 4, 2003 / 52(RR07);1-16, Recommendations for Using Smallpox Vaccine in a Pre-Event Vaccination Program, "During the interval in which vaccinia virus is shed, inadvertent inoculation can occur from the vaccination site to another area of the body, most commonly the face, eyelid, nose, lips, genitalia, or anus. In addition, transmission could occur to another nonimmune person, leading to self-limited infections or to more serious complications, particularly among persons with medical contraindications to vaccination. The risk for mortality from eczema vaccinatum might be higher among infected contacts than among vaccines." http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5207a1.htm
(16) Rotavirus, "Fecal shedding of vaccine virus was evaluated in a subset of persons enrolled in the phase III trials. Vaccine virus was shed by 9% of 360 infants after dose 1, but none of 249 and 385 infants after doses 2 and 3, respectively. Shedding was observed as early as 1 day and as late as 15 days after a dose. The potential for transmission of vaccine virus was not assessed." http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/rota.pdf
(17) DOI: 10.3201/eid1607.091606, Suggested citation for this article: Shahmahmoodi S, Mamishi S, Aghamohammadi A, Aghazadeh N, Tabatabaie H, Goya MM, et al. Vaccine-associated paralytic poliomyelitis in immunodeficient children, Iran, 1995–2008. Emerg Infect Dis. 2010 Jul; [Epub ahead of print], http://www.cdc.gov/eid/content/16/7/pdfs/09-1606.pdf
(18) Addition of Severe Combined Immunodeficiency as a Contraindication for Administration of Rotavirus Vaccine, MMWR, Weekly / Vol. 59 / No. 22 June 11, 2010, "The eight infants (four males and four females) were diagnosed with SCID between ages 3 months and 9 months and had received 1–3 doses of rotavirus vaccine before the diagnosis. All the infants had diarrhea, and most had additional infections (e.g., Pneumocystis jirovecii, rhinovirus, adenovirus, Salmonella, Escherichia coli, and Giardia) at the time of SCID diagnosis. Rotavirus infection was diagnosed by enzyme immunoassay in seven of the eight patients for whom this information was available. In all eight cases, vaccine-acquired rotavirus infection was confirmed by reverse transcription — polymerase chain reaction (RT-PCR) and nucleotide sequencing. Prolonged shedding of vaccine virus was documented in at least six of these cases, with duration of up to 11 months."
The report, published in today's issue of Morbidity and Mortality Weekly Report (MMWR), said the woman sought treatment at a public health clinic in Alaska in October 2006 for increasingly painful vaginal tears that she said were not caused by sexual abuse or trauma.
Vaccinia, a rare cutaneous and sometimes systemic reaction to smallpox vaccination that can occur in vaccinees or in close contacts of vaccinees, has been reported more frequently in the medical literature since the US military revived its long-discontinued smallpox vaccination program in late 2002. By June 2006 about 1.1 million US service members bound for the Middle East and other areas thought to be high-risk had been vaccinated.
In March a 2-year-old Indiana boy was hospitalized with severe eczema vaccinatum that he contracted from his father, a soldier who had recently received a smallpox shot.
In the woman's case, in early October 2006 clinicians swabbed one of her two vulvar lesions and diagnosed secondary candidiasis; however, her symptoms continued to worsen despite treatment with over-the-counter medication, the MMWR report said. A healthcare provider then diagnosed cellulitis, discontinued the over-the-counter remedy, and prescribed a 7-day course of oral cephalexin. The patient's symptoms resolved 1 week later.
In the meanwhile, the Alaska State Virology Laboratory (ASVL) found the woman did not have herpes simplex virus, but it was unable to identify what virus she had. About a month later, the virus remained unidentified after the ASVL submitted it to a reference lab.
On Jan 9, 2007, the ASVL submitted the unidentified isolate to the CDC, where cloning and sequencing of her sample, based on products from specialized polymerase chain reaction (PCR) testing, identified DNA bands that matched vaccinia virus sequences. Additional PCR testing performed by the CDC Poxvirus Laboratory revealed that the woman's isolate was consistent with a vaccine-strain vaccinia virus, and the lab relayed the results to the ASVL on Jan 30.
Upon receiving the woman's test result, Alaska state health officials interviewed the woman and learned that she had never been vaccinated against smallpox but that the only sex partner she had had from 1 month before her infection until the lesions were healed was a US service member. She said their intimate contact involved manual stimulation and vaginal intercourse, and she didn't remember seeing bandages or unusual skin lesions on her partner.
When state health officials interviewed medical officers at the military base, they found that the soldier had been deployed overseas in late October. He had received a smallpox vaccination on Sep 19, 2006, along with instruction on vaccination site care and proper hand hygiene. They found no other transmission of the virus from either the soldier or from the woman to other people, including the heathcare workers who examined her.
The US military revived its long-discontinued smallpox vaccination program in late 2002, and by June 2006 about 1.1 million service members had been vaccinated.
Contact vaccinia, as in the woman's case, is more common than the more severe eczema vaccinatum or progressive vaccinia. According to the US Department of Defense (DoD) Web site, 61 cases (36 lab-confirmed) of contact vaccinia occurred, mainly to spouses and adult intimate contacts, between Dec 13, 2002, and Apr 12, 2007. The MMWR report said lab-confirmed vulvar vaccinia after sexual contact with vaccinated military members has also been reported in New York and Texas since the DoD resumed its smallpox vaccination program.
The CDC and the DoD have received four reports of nongenital contact vaccinia since Mar 8, 2007, related to recently vaccinated service members, the MMWR report said.
In an editorial note that accompanied the report of the woman's case, the CDC wrote that to prevent contact vaccinia transfers, healthcare providers should educate vaccinees on proper handwashing after bandage changesas well as on how to avoid other contact with the vaccination site. It said asking patients who seek treatment for vesicular lesions that resemble vaccinia about contact with recent smallpox vaccines can speed diagnosis and provide quicker contact tracing, clinical intervention, and counseling about preventing further transmission.
CDC. Vulvar vaccinia infection after sexual contact with a military smallpox vaccine—Alaska, 2006. MMWR 2007 May 4;56(17):417-19 [Full text]
(doi:10.1542/peds.2009-1901)
Published online January 25, 2010
Case Reports
Sibling Transmission of Vaccine-Derived Rotavirus (RotaTeq) Associated With Rotavirus Gastroenteritis
Daniel C. Payne, PhD, MSPHa, Kathryn M. Edwards, MDb, Michael D. Bowen, PhDc, Erin Keckley, RNb, Jody Peters, MSb, Mathew D. Esona, PhDc, Elizabeth N. Teel, BSc, Diane Kent, RNb, Umesh D. Parashar, MBBS, MPHa, Jon R. Gentsch, PhDc
Although rotavirus vaccines are known to be shed in stools, transmission of vaccine-derived virus to unvaccinated contacts resulting in symptomatic rotavirus gastroenteritis has not been reported to our knowledge. We document here the occurrence of vaccine-derived rotavirus (RotaTeq [Merck and Co, Whitehouse Station, NJ]) transmission from a vaccinated infant to an older, unvaccinated sibling, resulting in symptomatic rotavirus gastroenteritis that required emergency department care. Results of our investigation suggest that reassortment between vaccine component strains of genotypes P7[5]G1 and P1A[8]G6 occurred during replication either in the vaccinated infant or in the older sibling, raising the possibility that this reassortment may have increased the virulence of the vaccine-derived virus. Both children remain healthy 11 months after this event and are without underlying medical conditions.
Key Words: rotavirus • acute gastroenteritis • RotaTeq • rotavirus vaccine • WC3 • sibling transmission • horizontal transmission • shedding • reassortant • New Vaccine Surveillance Network • NVSN
Abbreviations: RT-PCR, reverse transcription-polymerase chain reaction
Accepted Nov 20, 2009.
(15) MMWR, Recommendations and Reports, April 4, 2003 / 52(RR07);1-16, Recommendations for Using Smallpox Vaccine in a Pre-Event Vaccination Program, "During the interval in which vaccinia virus is shed, inadvertent inoculation can occur from the vaccination site to another area of the body, most commonly the face, eyelid, nose, lips, genitalia, or anus. In addition, transmission could occur to another nonimmune person, leading to self-limited infections or to more serious complications, particularly among persons with medical contraindications to vaccination. The risk for mortality from eczema vaccinatum might be higher among infected contacts than among vaccines." http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5207a1.htm
(16) Rotavirus, "Fecal shedding of vaccine virus was evaluated in a subset of persons enrolled in the phase III trials. Vaccine virus was shed by 9% of 360 infants after dose 1, but none of 249 and 385 infants after doses 2 and 3, respectively. Shedding was observed as early as 1 day and as late as 15 days after a dose. The potential for transmission of vaccine virus was not assessed." http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/rota.pdf
(17) DOI: 10.3201/eid1607.091606, Suggested citation for this article: Shahmahmoodi S, Mamishi S, Aghamohammadi A, Aghazadeh N, Tabatabaie H, Goya MM, et al. Vaccine-associated paralytic poliomyelitis in immunodeficient children, Iran, 1995–2008. Emerg Infect Dis. 2010 Jul; [Epub ahead of print], http://www.cdc.gov/eid/content/16/7/pdfs/09-1606.pdf
(18) Addition of Severe Combined Immunodeficiency as a Contraindication for Administration of Rotavirus Vaccine, MMWR, Weekly / Vol. 59 / No. 22 June 11, 2010, "The eight infants (four males and four females) were diagnosed with SCID between ages 3 months and 9 months and had received 1–3 doses of rotavirus vaccine before the diagnosis. All the infants had diarrhea, and most had additional infections (e.g., Pneumocystis jirovecii, rhinovirus, adenovirus, Salmonella, Escherichia coli, and Giardia) at the time of SCID diagnosis. Rotavirus infection was diagnosed by enzyme immunoassay in seven of the eight patients for whom this information was available. In all eight cases, vaccine-acquired rotavirus infection was confirmed by reverse transcription — polymerase chain reaction (RT-PCR) and nucleotide sequencing. Prolonged shedding of vaccine virus was documented in at least six of these cases, with duration of up to 11 months."
J Pediatr. 1997 Jul;131(1 Pt 1):151-4.
Transmission of varicella-vaccine virus from a healthy 12-month-old child to his pregnant mother.Salzman MB1, Sharrar RG, Steinberg S, LaRussa P.
Author information
AbstractA 12-month-old healthy boy had approximately 30 vesicular skin lesions 24 days after receiving varicella vaccine. Sixteen days later his pregnant mother had 100 lesions. Varicella-vaccine virus was identified by polymerase chain reaction in the vesicular lesions of the mother. After an elective abortion, no virus was detected in the fetal tissue. This case documents transmission of varicella-vaccine virus from a healthy 12-month-old infant to his pregnant mother.
Comment in
- Transmission of varicella-vaccine virus: what is the risk? [J Pediatr. 1998]
- Toddler-to-mother transmission of varicella-vaccine virus: how bad is that? [J Pediatr. 1997]
PMID: 9255208 [PubMed - indexed for MEDLINE]
Public Health Officials Know: Recently Vaccinated Individuals Spread Disease Tuesday, 3 Mar 2015 | 2:41 PM ETGlobe Newswire
Washington, D.C., March 3, 2015 (GLOBE NEWSWIRE) -- Physicians and public health officials know that recently vaccinated individuals can spread disease and that contact with the immunocompromised can be especially dangerous. For example, the Johns Hopkins Patient Guide warns the immunocompromised to "Avoid contact with children who are recently vaccinated," and to "Tell friends and family who are sick, or have recently had a live vaccine (such as chicken pox, measles, rubella, intranasal influenza, polio or smallpox) not to visit."1
A statement on the website of St. Jude's Hospital warns parents not to allow people to visit children undergoing cancer treatment if they have received oral polio or smallpox vaccines within four weeks, have received the nasal flu vaccine within one week, or have rashes after receiving the chickenpox vaccine or MMR (measles, mumps, rubella) vaccine.2
"The public health community is blaming unvaccinated children for the outbreak of measles at Disneyland, but the illnesses could just as easily have occurred due to contact with a recently vaccinated individual," says Sally Fallon Morell, president of the Weston A. Price Foundation. The Foundation promotes a healthy diet, non-toxic lifestyle and freedom of medical choice for parents and their children. "Evidence indicates that recently vaccinated individuals should be quarantined in order to protect the public."
Scientific evidence demonstrates that individuals vaccinated with live virus vaccines such as MMR (measles, mumps and rubella), rotavirus, chicken pox, shingles and influenza can shed the virus for many weeks or months afterwards and infect the vaccinated and unvaccinated alike. 3,4,5,6,7,8,9,10.11.12
Furthermore, vaccine recipients can carry diseases in the back of their throat and infect others while displaying no symptoms of a disease.13,14,15
Both unvaccinated and vaccinated individuals are at risk from exposure to those recently vaccinated. Vaccine failure is widespread; vaccine-induced immunity is not permanent and recent outbreaks of diseases such as whooping cough, mumps and measles have occurred in fully vaccinated populations.16,17 Flu vaccine recipients become more susceptible to future infection after repeated vaccination.18,19
Adults have contracted polio from recently vaccinated infants. A father from Staten Island ended up in a wheel chair after contracting polio while changing his daughter's diaper. He received a 22.5 million dollar award in 2009. 20,21
"Vaccine failure and failure to acknowledge that live virus vaccines can spread disease have resulted in an increase in outbreaks of infectious disease in both vaccinated and unvaccinated individuals," says Leslie Manookian, producer of The Greater Good. "CDC should instruct physicians who administer vaccinations to inform their patients about the risks posed to others by those who've been recently vaccinated."
According to the Weston A. Price Foundation, the best protection against infectious disease is a healthy immune system, supported by adequate vitamin A and vitamin C. Well-nourished children easily recover from infectious disease and rarely suffer complications.
The number of measles deaths declined from 7575 in 1920 (10,000 per year in many years in the 1910s) to an average of 432 each year from 1958-1962.22 The vaccine was introduced in 1963. Between 2005 and 2014, there have been no deaths from measles in the U.S. and 108 deaths reported after the MMR vaccine.23
The Weston A. Price Foundation is a 501(c)(3) nutrition education foundation with the mission of disseminating accurate, science-based information on diet and health. Named after nutrition pioneer Weston A. Price, DDS, author of Nutrition and Physical Degeneration, the Washington, DC-based Foundation publishes a quarterly journal for its 15,000 members, supports 600 local chapters worldwide and hosts a yearly international conference. The Foundation phone number is (202) 363-4394(202) 363-4394, www.westonaprice.org, info@westonaprice.org.
REFERENCES:
1. http://www.hopkinsmedicine.org/kimmel_cancer_center/patient_information/Patient%20Guide%20Final.pdf
2. http://www.stjude.org/stjude/v/index.jsp?vgnextoid=20206f9523e70110VgnVCM1000001e0215acRCRD
3. Outbreak of Measles Among Persons With Prior Evidence of Immunity, New York City, 2011 http://cid.oxfordjournals.org/content/early/2014/02/27/cid.ciu105
4. Detection of Measles Virus RNA in Urine Specimens from Vaccine Recipients http://www.ncbi.nlm.nih.gov/pubmed/7494055
5. Comparison of the Safety, Vaccine Virus Shedding and Immunogenicity of Influenza Virus Vaccine, Trivalent, Types A and B, Live Cold-Adapted, Administered to Human Immunodeficiency Virus (HIV)-Infected and Non-HIV Infected Adults http://jid.oxfordjournals.org/content/181/2/725.full
6. Sibling Transmission of Vaccine-Derived Rotavirus (RotaTeq) Associated with Rotavirus Gastroenteritis http://pediatrics.aappublications.org/content/125/2/e438
7. Polio vaccination may continue after wild virus fades http://www.cidrap.umn.edu/news-perspective/2008/10/polio-vaccination-may-continue-after-wild-virus-fades
8. Engineering attenuated virus vaccines by controlling replication fidelity http://www.nature.com/nm/journal/v14/n2/abs/nm1726.html
9. CASE OF VACCINE-ASSOCIATED MEASLES FIVE WEEKS POST-IMMUNISATION, BRITISH COLUMBIA, CANADA, OCTOBER 2013 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20649
10. The Safety Profile of Varicella Vaccine: A 10-Year Review http://jid.oxfordjournals.org/content/197/Supplement_2/S165.full
11. Comparison of Shedding Characteristics of Seasonal Influenza Virus (Sub)Types and Influenza A(H1N1)pdm09; Germany, 2007-2011 http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0051653
12. Epigenetics of Host-Pathogen Interactions: The Road Ahead and the Road Behind http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1003007
13. Animal Models for Influenza Virus Pathogenesis and Transmission http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3063653/
14. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate mode http://www.ncbi.nlm.nih.gov/pubmed/24277828
15. Study Finds Parents Can Pass Whooping Cough to Babies http://www.nytimes.com/2007/04/03/health/03coug.html?_r=0
16. Immunized People Getting Whooping Cough http://www.kpbs.org/news/2014/jun/12/immunized-people-getting-whooping-cough/
17. Vaccine Failure -- Over 1000 Got Mumps in NY in Last Six Months http://articles.mercola.com/sites/articles/archive/2010/03/06/vaccine-failure-over-1000-get-mumps-in-ny-in-last-six-months.aspx
18. Impact of Repeated Vaccination on Vaccine Effectiveness Against Influenza A(H3N2) and B During 8 Seasons http://cid.oxfordjournals.org/content/early/2014/09/29/cid.ciu680.full
19. http://articles.mercola.com/sites/articles/archive/2012/09/18/flu-shot-increases-flu-illness.aspx
20. http://www.nydailynews.com/new-york/staten-island-dad-22-5m-polio-case-lederle-laboratories-article-1.369105
21. http://naturalsociety.com/woman-contracts-polio-virus-vaccinated-infant/
22. http://www.cdc.gov/mmwr/preview/mmwrhtml/00056803.htm
23. http://vaccineimpact.com/2015/zero-u-s-measles-deaths-in-10-years-but-over-100-measles-vaccine-deaths-reported/
CONTACT: Kim Hartke, 703-860-2711703-860-2711, press@westonaprice.org Leslie Manookian, 208-721-2135208-721-2135, leslie@greatergoodmovie.org
Source:Weston A. Price Foundation
Washington, D.C., March 3, 2015 (GLOBE NEWSWIRE) -- Physicians and public health officials know that recently vaccinated individuals can spread disease and that contact with the immunocompromised can be especially dangerous. For example, the Johns Hopkins Patient Guide warns the immunocompromised to "Avoid contact with children who are recently vaccinated," and to "Tell friends and family who are sick, or have recently had a live vaccine (such as chicken pox, measles, rubella, intranasal influenza, polio or smallpox) not to visit."1
A statement on the website of St. Jude's Hospital warns parents not to allow people to visit children undergoing cancer treatment if they have received oral polio or smallpox vaccines within four weeks, have received the nasal flu vaccine within one week, or have rashes after receiving the chickenpox vaccine or MMR (measles, mumps, rubella) vaccine.2
"The public health community is blaming unvaccinated children for the outbreak of measles at Disneyland, but the illnesses could just as easily have occurred due to contact with a recently vaccinated individual," says Sally Fallon Morell, president of the Weston A. Price Foundation. The Foundation promotes a healthy diet, non-toxic lifestyle and freedom of medical choice for parents and their children. "Evidence indicates that recently vaccinated individuals should be quarantined in order to protect the public."
Scientific evidence demonstrates that individuals vaccinated with live virus vaccines such as MMR (measles, mumps and rubella), rotavirus, chicken pox, shingles and influenza can shed the virus for many weeks or months afterwards and infect the vaccinated and unvaccinated alike. 3,4,5,6,7,8,9,10.11.12
Furthermore, vaccine recipients can carry diseases in the back of their throat and infect others while displaying no symptoms of a disease.13,14,15
Both unvaccinated and vaccinated individuals are at risk from exposure to those recently vaccinated. Vaccine failure is widespread; vaccine-induced immunity is not permanent and recent outbreaks of diseases such as whooping cough, mumps and measles have occurred in fully vaccinated populations.16,17 Flu vaccine recipients become more susceptible to future infection after repeated vaccination.18,19
Adults have contracted polio from recently vaccinated infants. A father from Staten Island ended up in a wheel chair after contracting polio while changing his daughter's diaper. He received a 22.5 million dollar award in 2009. 20,21
"Vaccine failure and failure to acknowledge that live virus vaccines can spread disease have resulted in an increase in outbreaks of infectious disease in both vaccinated and unvaccinated individuals," says Leslie Manookian, producer of The Greater Good. "CDC should instruct physicians who administer vaccinations to inform their patients about the risks posed to others by those who've been recently vaccinated."
According to the Weston A. Price Foundation, the best protection against infectious disease is a healthy immune system, supported by adequate vitamin A and vitamin C. Well-nourished children easily recover from infectious disease and rarely suffer complications.
The number of measles deaths declined from 7575 in 1920 (10,000 per year in many years in the 1910s) to an average of 432 each year from 1958-1962.22 The vaccine was introduced in 1963. Between 2005 and 2014, there have been no deaths from measles in the U.S. and 108 deaths reported after the MMR vaccine.23
The Weston A. Price Foundation is a 501(c)(3) nutrition education foundation with the mission of disseminating accurate, science-based information on diet and health. Named after nutrition pioneer Weston A. Price, DDS, author of Nutrition and Physical Degeneration, the Washington, DC-based Foundation publishes a quarterly journal for its 15,000 members, supports 600 local chapters worldwide and hosts a yearly international conference. The Foundation phone number is (202) 363-4394(202) 363-4394, www.westonaprice.org, info@westonaprice.org.
REFERENCES:
1. http://www.hopkinsmedicine.org/kimmel_cancer_center/patient_information/Patient%20Guide%20Final.pdf
2. http://www.stjude.org/stjude/v/index.jsp?vgnextoid=20206f9523e70110VgnVCM1000001e0215acRCRD
3. Outbreak of Measles Among Persons With Prior Evidence of Immunity, New York City, 2011 http://cid.oxfordjournals.org/content/early/2014/02/27/cid.ciu105
4. Detection of Measles Virus RNA in Urine Specimens from Vaccine Recipients http://www.ncbi.nlm.nih.gov/pubmed/7494055
5. Comparison of the Safety, Vaccine Virus Shedding and Immunogenicity of Influenza Virus Vaccine, Trivalent, Types A and B, Live Cold-Adapted, Administered to Human Immunodeficiency Virus (HIV)-Infected and Non-HIV Infected Adults http://jid.oxfordjournals.org/content/181/2/725.full
6. Sibling Transmission of Vaccine-Derived Rotavirus (RotaTeq) Associated with Rotavirus Gastroenteritis http://pediatrics.aappublications.org/content/125/2/e438
7. Polio vaccination may continue after wild virus fades http://www.cidrap.umn.edu/news-perspective/2008/10/polio-vaccination-may-continue-after-wild-virus-fades
8. Engineering attenuated virus vaccines by controlling replication fidelity http://www.nature.com/nm/journal/v14/n2/abs/nm1726.html
9. CASE OF VACCINE-ASSOCIATED MEASLES FIVE WEEKS POST-IMMUNISATION, BRITISH COLUMBIA, CANADA, OCTOBER 2013 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20649
10. The Safety Profile of Varicella Vaccine: A 10-Year Review http://jid.oxfordjournals.org/content/197/Supplement_2/S165.full
11. Comparison of Shedding Characteristics of Seasonal Influenza Virus (Sub)Types and Influenza A(H1N1)pdm09; Germany, 2007-2011 http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0051653
12. Epigenetics of Host-Pathogen Interactions: The Road Ahead and the Road Behind http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1003007
13. Animal Models for Influenza Virus Pathogenesis and Transmission http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3063653/
14. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate mode http://www.ncbi.nlm.nih.gov/pubmed/24277828
15. Study Finds Parents Can Pass Whooping Cough to Babies http://www.nytimes.com/2007/04/03/health/03coug.html?_r=0
16. Immunized People Getting Whooping Cough http://www.kpbs.org/news/2014/jun/12/immunized-people-getting-whooping-cough/
17. Vaccine Failure -- Over 1000 Got Mumps in NY in Last Six Months http://articles.mercola.com/sites/articles/archive/2010/03/06/vaccine-failure-over-1000-get-mumps-in-ny-in-last-six-months.aspx
18. Impact of Repeated Vaccination on Vaccine Effectiveness Against Influenza A(H3N2) and B During 8 Seasons http://cid.oxfordjournals.org/content/early/2014/09/29/cid.ciu680.full
19. http://articles.mercola.com/sites/articles/archive/2012/09/18/flu-shot-increases-flu-illness.aspx
20. http://www.nydailynews.com/new-york/staten-island-dad-22-5m-polio-case-lederle-laboratories-article-1.369105
21. http://naturalsociety.com/woman-contracts-polio-virus-vaccinated-infant/
22. http://www.cdc.gov/mmwr/preview/mmwrhtml/00056803.htm
23. http://vaccineimpact.com/2015/zero-u-s-measles-deaths-in-10-years-but-over-100-measles-vaccine-deaths-reported/
CONTACT: Kim Hartke, 703-860-2711703-860-2711, press@westonaprice.org Leslie Manookian, 208-721-2135208-721-2135, leslie@greatergoodmovie.org
Source:Weston A. Price Foundation
Public Health Officials Know: Recently Vaccinated Individuals Spread Disease
Tuesday, 3 Mar 2015 | 2:41 PM ETGlobe News
http://www.cnbc.com/2015/03/03/globe-newswire-public-health-officials-know-recently-vaccinated-individuals-spread-disease.html
Washington, D.C., March 3, 2015 (GLOBE NEWSWIRE) -- Physicians and public health officials know that recently vaccinated individuals can spread disease and that contact with the immunocompromised can be especially dangerous. For example, the Johns Hopkins Patient Guide warns the immunocompromised to "Avoid contact with children who are recently vaccinated," and to "Tell friends and family who are sick, or have recently had a live vaccine (such as chicken pox, measles, rubella, intranasal influenza, polio or smallpox) not to visit."1
A statement on the website of St. Jude's Hospital warns parents not to allow people to visit children undergoing cancer treatment if they have received oral polio or smallpox vaccines within four weeks, have received the nasal flu vaccine within one week, or have rashes after receiving the chickenpox vaccine or MMR (measles, mumps, rubella) vaccine.2
"The public health community is blaming unvaccinated children for the outbreak of measles at Disneyland, but the illnesses could just as easily have occurred due to contact with a recently vaccinated individual," says Sally Fallon Morell, president of the Weston A. Price Foundation. The Foundation promotes a healthy diet, non-toxic lifestyle and freedom of medical choice for parents and their children. "Evidence indicates that recently vaccinated individuals should be quarantined in order to protect the public."
Scientific evidence demonstrates that individuals vaccinated with live virus vaccines such as MMR (measles, mumps and rubella), rotavirus, chicken pox, shingles and influenza can shed the virus for many weeks or months afterwards and infect the vaccinated and unvaccinated alike. 3,4,5,6,7,8,9,10.11.12
Furthermore, vaccine recipients can carry diseases in the back of their throat and infect others while displaying no symptoms of a disease.13,14,15
Both unvaccinated and vaccinated individuals are at risk from exposure to those recently vaccinated. Vaccine failure is widespread; vaccine-induced immunity is not permanent and recent outbreaks of diseases such as whooping cough, mumps and measles have occurred in fully vaccinated populations.16,17 Flu vaccine recipients become more susceptible to future infection after repeated vaccination.18,19
Adults have contracted polio from recently vaccinated infants. A father from Staten Island ended up in a wheel chair after contracting polio while changing his daughter's diaper. He received a 22.5 million dollar award in 2009. 20,21
"Vaccine failure and failure to acknowledge that live virus vaccines can spread disease have resulted in an increase in outbreaks of infectious disease in both vaccinated and unvaccinated individuals," says Leslie Manookian, producer of The Greater Good. "CDC should instruct physicians who administer vaccinations to inform their patients about the risks posed to others by those who've been recently vaccinated."
According to the Weston A. Price Foundation, the best protection against infectious disease is a healthy immune system, supported by adequate vitamin A and vitamin C. Well-nourished children easily recover from infectious disease and rarely suffer complications.
The number of measles deaths declined from 7575 in 1920 (10,000 per year in many years in the 1910s) to an average of 432 each year from 1958-1962.22 The vaccine was introduced in 1963. Between 2005 and 2014, there have been no deaths from measles in the U.S. and 108 deaths reported after the MMR vaccine.23
The Weston A. Price Foundation is a 501(c)(3) nutrition education foundation with the mission of disseminating accurate, science-based information on diet and health. Named after nutrition pioneer Weston A. Price, DDS, author of Nutrition and Physical Degeneration, the Washington, DC-based Foundation publishes a quarterly journal for its 15,000 members, supports 600 local chapters worldwide and hosts a yearly international conference. The Foundation phone number is (202) 363-4394(202) 363-4394, www.westonaprice.org, info@westonaprice.org.
REFERENCES:
1.http://www.hopkinsmedicine.org/kimmel_cancer_center/patient_information/Patient%20Guide%20Final.pdf
2. http://www.stjude.org/stjude/v/index.jsp?vgnextoid=20206f9523e70110VgnVCM1000001e0215acRCRD
3. Outbreak of Measles Among Persons With Prior Evidence of Immunity, New York City, 2011 http://cid.oxfordjournals.org/content/early/2014/02/27/cid.ciu105
4. Detection of Measles Virus RNA in Urine Specimens from Vaccine Recipients http://www.ncbi.nlm.nih.gov/pubmed/7494055
5. Comparison of the Safety, Vaccine Virus Shedding and Immunogenicity of Influenza Virus Vaccine, Trivalent, Types A and B, Live Cold-Adapted, Administered to Human Immunodeficiency Virus (HIV)-Infected and Non-HIV Infected Adults http://jid.oxfordjournals.org/content/181/2/725.full
6. Sibling Transmission of Vaccine-Derived Rotavirus (RotaTeq) Associated with Rotavirus Gastroenteritis http://pediatrics.aappublications.org/content/125/2/e438
7. Polio vaccination may continue after wild virus fades http://www.cidrap.umn.edu/news-perspective/2008/10/polio-vaccination-may-continue-after-wild-virus-fades
8. Engineering attenuated virus vaccines by controlling replication fidelity http://www.nature.com/nm/journal/v14/n2/abs/nm1726.html
9. CASE OF VACCINE-ASSOCIATED MEASLES FIVE WEEKS POST-IMMUNISATION, BRITISH COLUMBIA, CANADA, OCTOBER 2013 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20649
10. The Safety Profile of Varicella Vaccine: A 10-Year Review http://jid.oxfordjournals.org/content/197/Supplement_2/S165.full
11. Comparison of Shedding Characteristics of Seasonal Influenza Virus (Sub)Types and Influenza A(H1N1)pdm09; Germany, 2007-2011 http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0051653
12. Epigenetics of Host-Pathogen Interactions: The Road Ahead and the Road Behind http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1003007
13. Animal Models for Influenza Virus Pathogenesis and Transmission http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3063653/
14. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate mode http://www.ncbi.nlm.nih.gov/pubmed/24277828
15. Study Finds Parents Can Pass Whooping Cough to Babies http://www.nytimes.com/2007/04/03/health/03coug.html?_r=0
16. Immunized People Getting Whooping Cough http://www.kpbs.org/news/2014/jun/12/immunized-people-getting-whooping-cough/
17. Vaccine Failure -- Over 1000 Got Mumps in NY in Last Six Months http://articles.mercola.com/sites/articles/archive/2010/03/06/vaccine-failure-over-1000-get-mumps-in-ny-in-last-six-months.aspx
18. Impact of Repeated Vaccination on Vaccine Effectiveness Against Influenza A(H3N2) and B During 8 Seasons http://cid.oxfordjournals.org/content/early/2014/09/29/cid.ciu680.full
19. http://articles.mercola.com/sites/articles/archive/2012/09/18/flu-shot-increases-flu-illness.aspx
20. http://www.nydailynews.com/new-york/staten-island-dad-22-5m-polio-case-lederle-laboratories-article-1.369105
21. http://naturalsociety.com/woman-contracts-polio-virus-vaccinated-infant/
22. http://www.cdc.gov/mmwr/preview/mmwrhtml/00056803.htm
23. http://vaccineimpact.com/2015/zero-u-s-measles-deaths-in-10-years-but-over-100-measles-vaccine-deaths-reported/
CONTACT: Kim Hartke, 703-860-2711703-860-2711, press@westonaprice.org Leslie Manookian, 208-721-2135208-721-2135, leslie@greatergoodmovie.org
Source:Weston A. Price Foundation